A recent report highlights interim findings in assessing the safety and effectiveness of Omnitrope® in adults aged 30 years or above lacking growth hormone. The "Italian Interim Snapshot" report aims to understand the ongoing safety and effectiveness of Omnitrope®, a recombinant growth hormone identical to Genotropin®, among Italian adults participating in the PATRO Adults study suffering from growth hormone deficiency (GHD).
Methods
The PATRO Adults study is a long-term observational non-interventional international post-marketing research project in many European nations. The primary objective is to understand long-term safety, while the secondary objective is to focus on the effectiveness of Omnitrope®. The study evaluated the drug's effectiveness using measures like bone mineral density, serum insulin-like growth factor-1 levels, lipid levels, and body composition. This report presents findings from the study's Italian participants.
Results
The study involved 67 adult patients with an average age of around 50 years, and 61% of them were males. These patients were treated with Omnitrope® over an average period of about 45.4 months, although the duration of treatment for some individuals varied by around 24.3 months. Approximately 55% of the patients noticed adverse effects during the treatment, including problems like abdominal distension, joint pain, decreased sensation, and muscle pain. To be specific, 4.5% of the subjects had adverse reactions directly associated with the drug. However, 14 patients reported adverse events, none of which were directly linked to Omnitrope®. When comparing the effectiveness of Omnitrope® with other similar medications, it was found to be equally effective.
Overall, while a significant portion of patients experienced adverse events during treatment, Omnitrope® demonstrated comparable effectiveness to other treatments without any direct attribution to adverse events among the patients.
Conclusions
This research confirms that Omnitrope® is effective and safe for Italian adults suffering from growth hormone deficiency (GHD). Given the small number of participants, these findings must be validated through the entire PATRO Adults study conducted globally. It also needs to be noted here that the treatment's effectiveness varies from individual to individual and is not a magical cure or a secret to eternal life.
Introduction
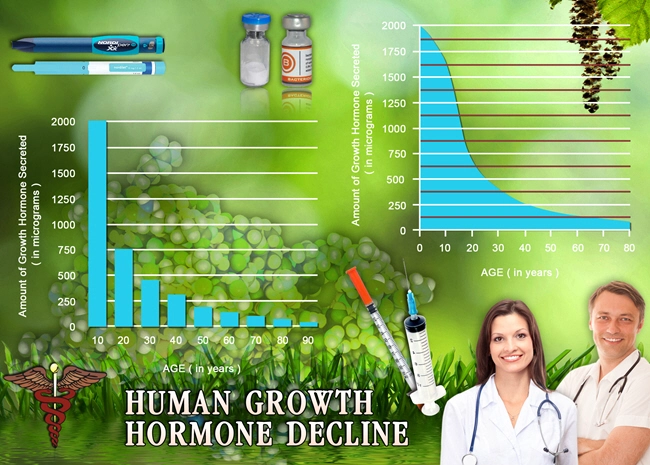
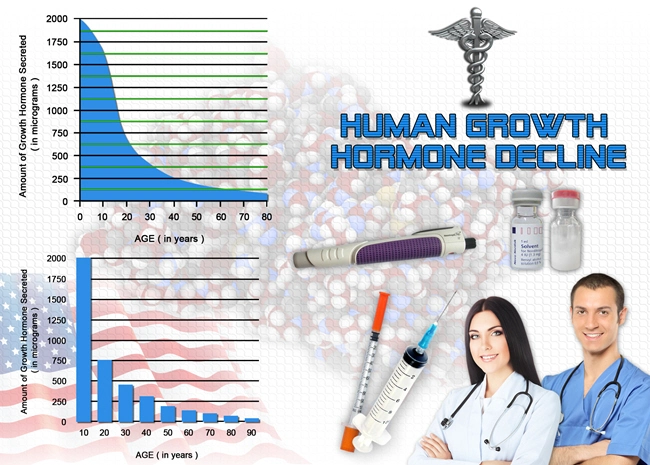
When the pituitary gland in adults does not produce enough growth hormone (GH), they develop a medical condition called adult-onset growth hormone deficiency (GHD). The condition is characterized by abnormal body composition, substrate metabolism, and physical and psychological functioning. Adult-onset GHD leads to problems like increased body fat, especially in the abdomen. The condition makes it difficult to control their body weight and decreases muscle mass, resulting in minimized muscle strength and exercise capacity. It also affects the mental well-being of the person, leading to higher anxiety levels and depression that impact their overall quality of life. Adult-onset GHD occurs due to hypothalamic-pituitary disease, head injuries, surgeries, and radiation treatments received for brain and pituitary tumors.
The treatment of GHD in adults is recommended with a replacement therapy that involves the use of recombinant human GH (rhGH) or synthesized human growth hormone. The dosage is based on specific blood test results called IGF-1 or plasma insulin-like growth factor-1 and the kind of side effects in the person. The therapy helps fix abnormalities related to metabolism, functions, and psychology linked to adult GHD. At the same time, lipolysis or reduction in total body fat, especially in the abdominal region, increases during the replacement therapy. One year of rhGH therapy has been shown to improve the heart from early damage in adult patients with GHD. Evidence suggests that the treatment can boost adult patients' ability to exercise and physical performance.
Omnitrope® (Sandoz, Kundl, Austria) is one of the most effective recombinant human growth hormone (rhGH) therapies used in treating growth hormone deficiency (GHD) in adults. Omnitrope® is produced by genetically modifying an Escherichia coli strain. There is a similarity between Omnitrope® and Genotropin® (Pfizer Limited, Sandwich, UK), while the former was the first to get approval from the European Medicines Agency (EMA) in 2006, which was a significant milestone within the European biosimilar regulatory framework.
Omnitrope® is approved for use in adult-onset growth hormone deficiency (GHD). Research involving adult patients indicates that the shift from Genotropin® to Omnitrope® does not affect the safety or efficacy. Nevertheless, there is a lack of data on the long-term safety and effectiveness of Omnitrope® for use in everyday clinical settings. The Patients Treated with Omnitrope® (PATRO) Adults study was launched to address this gap, with its beginning seeded in the Omnitrope® Active Pharmacovigilance Program established in agreement with the European Medicines Agency (EMA) following the approval of Omnitrope®. The PATRO Adults study is a comprehensive long-term post-marketing surveillance (PMS) project that evaluated Omnitrope®'s long-term safety and effectiveness in adult patients with GHD. The initiative was taken as a part of the Risk Management Plan for Omnitrope®, which was designed to meet the EMA's requirements.
The project's Early findings suggest that Omnitrope® can be generally well tolerated in standard clinical practice. Between September 2007 and September 2015, the PATRO Adults study featured 954 patients from eight countries (the Czech Republic, France, Germany, Italy, Spain, Sweden, The Netherlands, and the UK). This report focuses on the results of patients from Italy.
Treatment and Outcomes
Participants in the study were administered Omnitrope® as per the guidelines outlined in the Summary of Product Characteristics and the prescribed information relevant to each country. The main goal of this post-marketing surveillance (PMS) study was to collect and analyze information related to the long-term safety of Omnitrope® when used in a standard clinical setting. In other words, it is essential to understand the comprehensive global safety profile of Omnitrope®. It specifically stresses safety concerns, for example, the potential risk of developing glucose intolerance or diabetes or increased chances of cancer, and its effect on cardiovascular risk factors like blood pressure and levels of inflammatory markers. The results have been segregated and incorporated in the Sandoz Safety Database as Adverse Events (AEs), Adverse Drug Reactions (ADRs), Serious Adverse Events (SAEs), and Serious Adverse Drug Reactions (SADRs).
The next goal of the study was to observe the subjects who had received the treatment and gather data on the effectiveness of the Omnitrope® treatment. Measures like IGF-1 change levels per the normal ranges, depending on age and gender. It was also about understanding the fasting lipid profile and assessing body composition metrics like weight, waist and hip circumference, lean and total body mass, and body mass index.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Our HGH Clinic And Web Site Privacy Policy [Last Updated On: May 23rd, 2019] [Originally Added On: December 14th, 2017]
- Idaho HGH Clinics [Last Updated On: December 22nd, 2023] [Originally Added On: March 19th, 2018]
- Injectable HGH Prescriptions In Cheyenne, Wyoming [Last Updated On: September 19th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Milwaukee, Wisconsin [Last Updated On: October 4th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Madison, Wisconsin [Last Updated On: August 25th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Green Bay, Wisconsin [Last Updated On: August 1st, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Charleston, West Virginia [Last Updated On: November 29th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Vancouver, Washington [Last Updated On: May 25th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Tacoma, Washington [Last Updated On: March 12th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Spokane, Washington [Last Updated On: September 27th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Seattle, Washington [Last Updated On: December 27th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Washington D.C [Last Updated On: March 10th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Bellevue, Washington [Last Updated On: January 2nd, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Virginia Beach, Virginia [Last Updated On: March 16th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Richmond, Virginia [Last Updated On: March 30th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Portsmouth, Virginia [Last Updated On: September 13th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Norfolk, Virginia [Last Updated On: December 24th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Newport News, Virginia [Last Updated On: September 16th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Hampton, Virginia [Last Updated On: February 26th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Chesapeake, Virginia [Last Updated On: February 16th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Arlington, Virginia [Last Updated On: April 19th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Alexandria, Virginia [Last Updated On: December 13th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Montpelier, Vermont [Last Updated On: October 20th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In West Valley City, Utah [Last Updated On: September 26th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In West Jordan, Utah [Last Updated On: July 17th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Salt Lake City, Utah [Last Updated On: April 5th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Provo, Utah [Last Updated On: November 19th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Wichita Falls, Texas [Last Updated On: August 27th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Waco, Texas [Last Updated On: July 19th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In San Antonio, Texas [Last Updated On: April 10th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Round Rock, Texas [Last Updated On: January 21st, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Richardson, Texas [Last Updated On: July 1st, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Plano, Texas [Last Updated On: April 15th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Pasadena, Texas [Last Updated On: August 24th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Midland, Texas [Last Updated On: October 10th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Mesquite, Texas [Last Updated On: September 12th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In McKinney, Texas [Last Updated On: December 4th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In McAllen, Texas [Last Updated On: August 29th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Lubbock, Texas [Last Updated On: November 14th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Lewisville, Texas [Last Updated On: April 6th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Laredo, Texas [Last Updated On: October 15th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Killeen, Texas [Last Updated On: June 25th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Irving, Texas [Last Updated On: May 6th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Houston, Texas [Last Updated On: September 4th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Grand Prairie, Texas [Last Updated On: March 15th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Garland, Texas [Last Updated On: May 16th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Fort Worth, Texas [Last Updated On: October 6th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In El Paso, Texas [Last Updated On: June 20th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Denton, Texas [Last Updated On: November 6th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Dallas, Texas [Last Updated On: January 25th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Corpus Christi, Texas [Last Updated On: January 12th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Carrollton, Texas [Last Updated On: January 7th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Brownsville, Texas [Last Updated On: April 9th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Beaumont, Texas [Last Updated On: August 30th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Austin, Texas [Last Updated On: January 10th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Arlington, Texas [Last Updated On: March 4th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Amarillo, Texas [Last Updated On: July 22nd, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Abilene, Texas [Last Updated On: February 4th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Nashville, Tennessee [Last Updated On: June 22nd, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Murfreesboro, Tennessee [Last Updated On: June 28th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Memphis, Tennessee [Last Updated On: August 15th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Knoxville, Tennessee [Last Updated On: August 8th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Clarksville, Tennessee [Last Updated On: April 7th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Chattanooga, Tennessee [Last Updated On: April 6th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Sioux Falls, South Dakota [Last Updated On: January 16th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Columbia, South Carolina [Last Updated On: April 7th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Charleston, South Carolina [Last Updated On: April 7th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Providence, Rhode Island [Last Updated On: April 7th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Pittsburgh, Pennsylvania [Last Updated On: December 3rd, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Erie, Pennsylvania [Last Updated On: February 2nd, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Allentown, Pennsylvania [Last Updated On: November 4th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Salem, Oregon [Last Updated On: April 7th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Portland, Oregon [Last Updated On: April 7th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Gresham, Oregon [Last Updated On: April 7th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Eugene, Oregon [Last Updated On: April 4th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Tulsa, Oklahoma [Last Updated On: December 15th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Oklahoma City, Oklahoma [Last Updated On: June 6th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Norman, Oklahoma [Last Updated On: October 19th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Toledo, Ohio [Last Updated On: September 17th, 2023] [Originally Added On: March 3rd, 2019]
- Injectable HGH Prescriptions In Dayton, Ohio [Last Updated On: September 15th, 2023] [Originally Added On: March 3rd, 2019]