Jeff Bezos Wants to Stop Aging!
Undoubtedly, Jeff Bezos has shaken up the world. From his initial success of starting an Internet book store in a rented garage (a visionary leap that was  ridiculed and mocked by many) that turned into the money-making juggernaut of Amazon to founding Blue Origin, a human spaceflight startup, to purchasing the Washington Post, funding the Bezos Earth Fund, and his countless investments made through his venture capital firm Bezos Expeditions, and co-founding Altos Labs, a biotechnology company, Bezos is indeed a man of action. He has had the Midas touch in all of his endeavors.
ridiculed and mocked by many) that turned into the money-making juggernaut of Amazon to founding Blue Origin, a human spaceflight startup, to purchasing the Washington Post, funding the Bezos Earth Fund, and his countless investments made through his venture capital firm Bezos Expeditions, and co-founding Altos Labs, a biotechnology company, Bezos is indeed a man of action. He has had the Midas touch in all of his endeavors.
But has he finally met his match?
Jeff has entered the arena and climbed into the ring to take on an undefeated opponent. Glaring at him from across the ring is an adversary that is not only undefeated; he has fatally destroyed every challenger that has tried to evade him: “Old-Man Aging.”
That’s right. Jeff Bezos plans to knock out Old Man Aging. To help him in this mission, he has teamed up with Hal Barron, formerly with GlaxoSmithKline, to help lead Altos Labs, the aspiring, determined, new longevity company with billions of investment capital.
lead Altos Labs, the aspiring, determined, new longevity company with billions of investment capital.
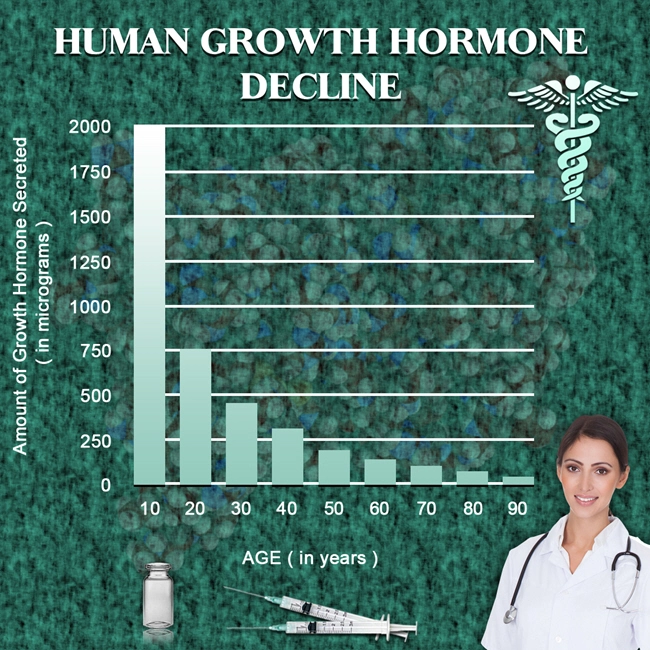
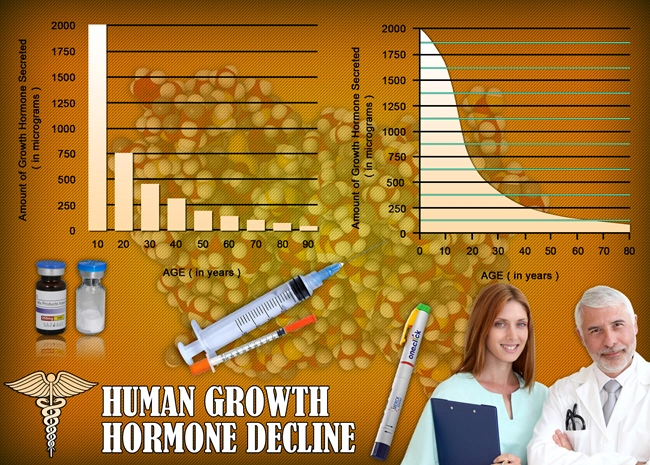
So what do the scientists and researchers say? Is there any chance that we could upset the monstrous foe of aging? Aging is far from superficial; the real action of aging occurs at deep cellular levels. In a lab petri dish, adult skin cells divide around 50 times before quitting. But skin cells from a newborn baby can divide 80 or 90 times. By contrast, cells from an elderly person divide only around 20 times.
Aging also has a substantial genetic component. Our genes constantly change as time passes due to chemicals that are attached and change which genes are switched on or off. These are called epigenetic changes, and they increase as we age.
Another type of change occurs at the tips of our cell’s DNA. Repeating sections of DNA called telomeres act like the plastic tip of a shoelace, blocking the twisted coils of genetic material from unraveling at the ends or binding together. Here’s the problem: telomeres shorten every time a cell divides. It’s unknown if short telomeres are simply signs of aging, like wrinkles, an expanding mid-section, and a loss of hair, or are part of how cells age. The length of telomeres is one of the keys to the aging puzzle.
To stay alive and continue dividing, immune cells stop their telomeres from shortening as they multiply, like cancer cells. This arguably has a hand in their seeming immortality. Drugs that stop telomerase from working also demonstrate hope against cancer (although cancer cells are slippery and can alter to evade attempts to kill them). Since aging significantly affects our cells and genes, a much larger question emerges: Why does this happen? Why do we age?
In the past, the idea was that aging occurred for the continuing evolution of species since evolution requires an “out-with-the-old, in-with-the-new” rotation of individuals. However, one problem with this concept is that most creatures do not attain old age. Most animals are killed by predators, disease, climate, or starvation. So an intrinsic limit on an animal’s lifespan may not be crucial to evolution.
Another idea is that aging results from the damage that builds up over time caused by the accumulation of minor injuries, stress, and fatigue, the so-called “wear-and-tear” cause of aging. We are confident that genes weaken as we age, but it is not conclusively linked to aging.
As we age, some of the body’s cells become senescent, when the cell hangs around but stops dividing. Senescent cells accumulate in the body over a lifetime  and have both good and bad effects.
and have both good and bad effects.
They are beneficial because they secrete chemicals that help repair damaged tissue. However, as we age, senescent cells increase in number and disturb the typical structure of organs and tissues by emitting harmful toxins. A good analogy is a grumpy old man sitting alone in a gathering and muttering mean, nasty utterances to no one in particular.
Senescent cells might cause many of the afflictions we associate with aging. Mice in which senescent cells were removed aged at a much slower, healthier rate.
In recent decades we have learned a lot about aging: hormone depletion, cellular senescence, damage from free radicals, telomere shortening, DNA damage, unfolded proteins, oxidative stress, mitochondrial dysfunction, stem cell exhaustion, stress, disease, inflammation, environmental damage, glycation, and good old fashioned wear and tear.
But the fundamental mystery of why we age is still open. As mentioned above, aging is a multi-dimensional puzzle resulting in a hybrid dread of destruction, misery, pain, sickness, feebleness, and death.
It remains uncertain whether Bezos’ company can succeed in controlling this demon. But the good news is that our knowledge concerning the disease of aging is exploding by leaps and bounds. We are on the verge of some gigantic breakthroughs in this life-and-death struggle, and it will be fascinating to see what comes out of Bezos’ efforts.
Reference
https://www.thedailybeast.com/jeff-bezos-wants-to-stop-aging-what-does-that-even-mean
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Finally! A Safe, Effective and Easy Way to Slow Aging: Genotropin HGH Product [Last Updated On: February 17th, 2025] [Originally Added On: June 24th, 2020]
- Metformin: The First Effective Anti-Aging Drug? [Last Updated On: February 16th, 2025] [Originally Added On: June 29th, 2020]
- A new study says Human Growth Hormone (HGH) can reverse aging! [Last Updated On: October 13th, 2024] [Originally Added On: June 30th, 2020]
- What Metabolism Reveals About Aging and Mortality [Last Updated On: February 17th, 2025] [Originally Added On: December 1st, 2020]
- Stanford University: A Way to Reverse Age-Related Health Issues is a Real Possibility [Last Updated On: August 15th, 2025] [Originally Added On: February 4th, 2021]
- The Six Biggest Hormone Disruptors to Avoid [Last Updated On: October 8th, 2025] [Originally Added On: May 16th, 2021]
- Laugh It Up! Boost Your HGH FAST [Last Updated On: August 14th, 2025] [Originally Added On: June 7th, 2021]
- Why Poor Sleep Quality is a Serious Detriment to Your Health [Last Updated On: October 6th, 2025] [Originally Added On: June 12th, 2021]
- Scientists Bold Prediction: We'll Soon Extend Life Well Beyond 120” [Last Updated On: June 1st, 2025] [Originally Added On: August 24th, 2021]
- Can My Hormones Make Me Fat? [Last Updated On: August 13th, 2025] [Originally Added On: September 1st, 2021]
- Testosterone Therapy may protect Telomeres and slow aging [Last Updated On: February 16th, 2025] [Originally Added On: September 14th, 2021]
- Minoxidil: A potential cure for baldness? [Last Updated On: October 7th, 2024] [Originally Added On: November 16th, 2022]
- The Quest for the Secret of Longevity [Last Updated On: September 30th, 2025] [Originally Added On: November 24th, 2022]
- Is it possible to live to 180? Perhaps! [Last Updated On: October 19th, 2024] [Originally Added On: November 29th, 2022]