Video Link: https://vimeo.com/288896651
Video Download: Click Here To Download Video
Video Stream: Click Here To Stream Video
Video Link: https://vimeo.com/288896756
Video Download: Click Here To Download Video
Video Stream: Click Here To Stream Video
Video Link: https://vimeo.com/288896885
Video Download: Click Here To Download Video
Video Stream: Click Here To Stream Video
Video Link: https://vimeo.com/288896998
Video Download: Click Here To Download Video
Video Stream: Click Here To Stream Video
Acidosis: The Hidden Health Destroyer
Your Most Important AND Little Known Measure of Good Health
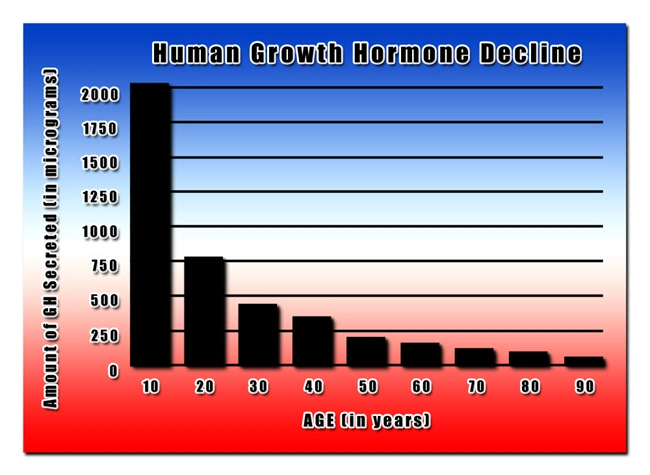
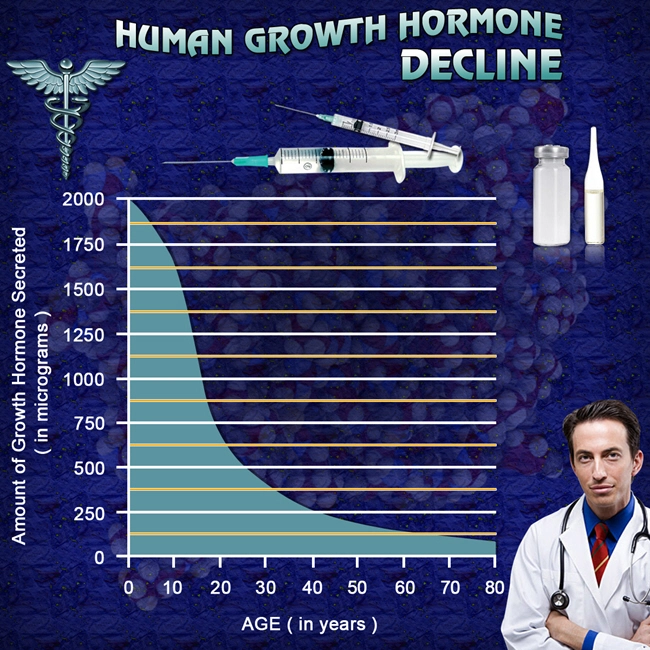
Human Growth Hormone (HGH) Replacement Therapy rejuvenates your entire body, both inside and out. Our growth hormone treatment regimens include a comprehensive approach that includes the most modern, effective, and safe methods of hormone replacement. But we don't stop there. We provide detailed recommendations on additional steps you can take to get and stay healthy, including the importance of avoiding acidosis.
What is the most important thing you should focus on for good health? Hint: it's not your weight...or your BMI...or your cholesterol...or your triglycerides...or even your blood pressure. Without a doubt, these numbers are significant. But there is one number that tells you more than these about your health, and you need to be aware of it to ward off disease and live pain-free:
Your Blood pH Level
The degree to which your blood is either acidic or alkaline in the body is potential hydrogen, or pH, which is expressed in a number between 0 and 14 and is usually measured via taking blood samples. A reading of 0 is pure acid, seven is neutral, and 14 is pure alkaline. Without exception, all of your organs, bones, tissues, and joints function at their best when your blood pH is slightly alkaline, which is around 7.35.
Here's a Brief Look at the Science Behind Acidosis

The stomach has an average pH level of approximately 4.0. Drinking alkaline water causes stomach pH to increase as it becomes more alkaline. When the stomach pH rises above 4.5, the stomach produces more hydrochloric acid. The hydrochloric acid formation chemical formula:
H20 + CO2 + NaCl = HCI =NaHCO3
This formula tells us that water, carbon dioxide, and sodium chloride generate hydrochloric acid and sodium bicarbonate. This hydrochloric acid goes into the gut, while the sodium bicarbonate travels into the blood.
The critical thing to remember is this: sodium bicarbonate works as an alkaline buffer in our veins. When the blood becomes too acidic, the alkaline buffer works to raise the pH. If we don't have enough sodium bicarbonates in our veins, we have trouble controlling the excess acid, which can result in premature aging, osteoporosis, high blood pressure, kidney stones, and a plethora of many other health problems.
For optimum health, the body's blood chemistry needs to be slightly alkaline, with a pH level ideally between 7.38 and 7.42. When the pH level strays too far above or below this level, disease and illness are likely to follow.
The important thing here is hydrogen ions (abbreviated with the chemical symbol H+). In water (H2O), a small number of the molecules separate. Some of the water molecules lose hydrogen and become hydroxide ions (OH¯). The missing hydrogen ions join up with water molecules to form hydronium ions (H3O+). To keep things simple, hydronium ions are referred to as hydrogen ions H+. In pure water, there is an equal number of hydrogen ions and hydroxide ions. The solution is neither acidic nor basic.
An acid is a component that contributes to hydrogen ions. As a result, when an acid is dissolved in water, the balance between hydrogen ions and hydroxide ions is shifted. Now, hydrogen ions outnumber hydroxide ions in the solution. The result is an acidic solution.
A base is a substance that accepts hydrogen ions. When a base is dissolved in water, the balance between hydrogen ions and hydroxide ions shifts the opposite way. Since the base absorbs hydrogen ions, the result is a solution with more hydroxide ions than hydrogen ions. This is an alkaline solution.
Acidity and alkalinity are measured with a logarithmic scale called pH. Logarithmic is defined as a number that shows how many times a base number (such as ten) is multiplied by itself to produce a third number (such as 100). Here is why a logarithmic scale is used: a strongly acidic solution can have one hundred million million (100,000,000,000,000) times more hydrogen ions than a strongly basic solution. Conversely, a strongly basic solution can have 100,000,000,000,000 times more hydroxide ions than a strongly acidic solution. Also, the hydrogen ion and hydroxide ion concentrations in common solutions can vary over that entire range.
To make things simpler, scientists use a logarithmic scale, the pH scale. Each one-unit change in the pH scale corresponds to a ten-fold change in hydrogen ion concentration. The pH scale is theoretically open-ended, but most pH values are in the range from 0 to 14. It's a lot easier to use a logarithmic scale instead of always having to write down all those zeros. Check out how one hundred million million is a one with fourteen zeros after it? It is not a coincidence; it is logarithms.
To be more precise, pH is the negative logarithm of the hydrogen ion concentration:
pH = -log [H+]
The square brackets around the H+ mean "concentration" to a chemist. What the equation means is what was mentioned earlier: for each 1-unit change in pH, the hydrogen ion concentration changes ten-fold.
To dispense with the science, here's what you need to remember: a can of the average soft drink has a pH of 3...a GIGANTIC 10,000 TIMES MORE ACIDIC THAN WATER! Something to consider when you reach for that next “refreshing pause.”
 Here's where things get interesting. As the number of hydrogen ions in a physical entity increases so does its level of acidity. In other words, acids emit hydrogen ions. A high concentration of hydrogen ions = an extremely acidic substance, which throws your pH balance way out-of-whack.
Here's where things get interesting. As the number of hydrogen ions in a physical entity increases so does its level of acidity. In other words, acids emit hydrogen ions. A high concentration of hydrogen ions = an extremely acidic substance, which throws your pH balance way out-of-whack.
The chemical representation of water is H2O. Hydrogen and oxygen are the two most significant components of water. Hydrogen ions are a part of water's chemical reaction, and the higher the concentration of hydrogen ions, the more acidic the water.
This pH balance is critical, and despite the typical American diet being acidic via environmental toxins, smoking, and a lack of exercise, it is possible to become too alkaline (known as alkalosis). However, a condition called acidosis (elevated acid level) is more common.
Acidosis is defined as too much acid in the body, a distinctly abnormal condition resulting from the accumulation of acid or the depletion of alkaline reserves. In acidosis, the pH of the blood is abnormally low.
Acidosis has medical symptoms such as confusion, coma, headaches, difficulty breathing, higher heart rate, nausea, muscular weakness, risk of seizures, and other serious problems.
Why Is This So Important?

Very simple: when your pH level is even slightly acidic, things can begin to go wrong. Aches and pains become worse, and diseases and other afflictions can gain a foothold. For example:
The heart: Of all the organs, the heart is the most susceptible to environmental pollutants and premature aging. If there are acidic toxins in the blood, the heart is forced to work harder. Also, acid wastes in the system rob the blood of oxygen, which can cause heart tissue to deteriorate. It's a simple explanation: the heart needs oxygen. Little oxygen = acidosis.
The liver: The liver is responsible for several hundred vital functions, and two of them are removing acid wastes from the blood and making alkaline enzymes for the body. Considering the typical American diet, a long with the environmental toxins we are constantly barraged with, the liver has to work hard to keep things on track. If this goes on too long, the liver may quit – and so will you
The Kidneys: The kidneys help get rid of excess acid from the body. If the blood is too acidic, the kidneys are overstressed, which can lead to serious health consequences
The digestive system: Foods that are acidic are hard for the stomach to break down, and the result is that the stomach may over-produce acid. This causes poor digestion, fewer nutrients being absorbed, and clogging the colon walls
Accelerated aging. An acidic environment makes your body less efficient, weaken cells, and accelerates the aging process
Fatigue. This can be explained by a simple reason: acidosis reduces the body's supply of oxygen, which is a primary source of energy
Loss of minerals. This unfortunate side effect of acidosis is a result of the body being forced to use its alkaline mineral supplies to neutralize excess acids in your fluids and tissues. Over time, this can have terrible and severe effects on the organs, as well as brittle nails, dry skin, sensitive gums, and thinning hair
Brain fog. The brain requires approximately 20% of all the oxygen you take in. As mentioned above, acidosis reduces the body's supply of oxygen. This may result in poor concentration, forgetfulness, and a host of other brain issues from mood swings to sleep impairment
Cancer. Two primary causes of cancer are an oxygen deficiency in the cells and tissues – and free radical damage. Alkalized, ionized water both boosts oxygenation and reduces oxidation. This suppresses unhealthy cells from pillaging the body. Consequently, when the body is in an alkaline state, there is robust resistance to the growth and proliferation of cancer cells
If you have these problems, there is a possibility that you are too acidic. Many people are affected, despite solutions to this dangerous condition...solutions that are familiar and easy to put to use.
The One Time an Acidic pH Level is Average
If you're still not convinced that acidosis is a serious health issue, think about this: the one time an acidic pH is average is when you're DEAD! This is the only time that an acidic pH level is what nature intended. Why? So the body can decompose.
Another Reason to be Concerned With Your pH Level
The chances of Disease growing in an acidic environment and is less likely in an alkaline environment. In an acidic environment, we are making our bodies work much harder, due to lower efficiency. This explains why disease can grow in an acidic environment to cause illness.
A slightly alkaline body is necessary for optimum health. Many diseases cannot survive in an alkaline environment.
You will never achieve optimum health in an acidic environment.
Also, when the body is in this condition, it will lose much of its ability to rid itself of toxins. This will increase the chance of forcing you to rely on antibiotics and expose you to a greater risk of nightmarish “superbugs” (a strain of bacteria that has become resistant to antibiotic drugs).
So Let's Do Something About This Problem
But enough talk about the problems due to an acidic pH level. Let's discuss ways to avoid these problems.
The key to getting your acid/alkaline ratio in balance is to make small changes to your diet and lifestyle. Nothing drastic...just add more alkaline foods to your diet, make sure your drinking water is not acidic and start feeling better and better each day.
In fact, as your body becomes more alkaline, your taste buds will change. This allows you to begin to taste the natural flavor of foods and make eating correctly far easier than you could ever have imagined.
Another benefit that occurs when the acid-alkaline balance is restored is this: once the body begins to move away from acidosis, it will start to detoxify by flushing out those acidic poisons. You will see and feel the difference with boundless energy, glowing skin, glistening hair, and losing pounds of unsightly, disease-producing fat.
You Are What You Eat, so Choose the Right Foods

As with all matters concerning health, so much begins with proper, smart nutrition. Since the conventional American diet consists mainly of acidic foods (meat, dairy products, grains, and sweets) and toxins in the environment, most people have an acidic pH level, and are well on the road to acidosis, with all of the dreaded consequences of that debilitating condition.
Therefore, step one in restoring your body to its proper pH balance is to make wise food selections. It is important to be aware of the acid/alkaline content of food. Here is a brief break down of acid and alkaline foods that help or hurt:
Acid Forming Foods:
- Corn Tortillas
- Sourdough Bread
- White Bread
- Ketchup
- Mayonnaise
- Mustard
- Cheese
- Eggs
- Milk (pasteurized)
- Yogurt (unsweetened)
- Beer
- Coffee
- Fruit juice
- Liquor
- Beef
- Pork
- Sardines (canned)
- Tuna (canned)
- Veal
- Artificial sweeteners
- Pickled vegetables
- Soda
Alkaline Forming Foods:
- Alfalfa
- Barley Grass
- Cucumber, fresh
- Kale
- Avacado
- Lima Beans
- White (navy) beans
- Baking Soda
- Fresh red beet
- Red Radish
- Cabbage
- Fresh Lettuce
- Celery
- Fresh cut green beans
- Garlic
- Ginger
- Oregano
- Spinach
It is, of course, nearly impossible to eat like a machine. Following the above list to a T is not only impossible but not needed. The key is first to become aware of acidic and alkaline foods, then begin to make a gradual shift to the alkaline side of the equation.
Hydration: Another All-Important Weapon in the Battle Against Acidosis
Drinking water that is acidifying can cause or worsen acidosis, which can lead to many serious health issues. Much of ordinary tap water is acidic, due to external contaminants (chemical treatment, pollution, and trace amounts of pharmaceuticals) and tap water's chemical makeup of hydrogen ions.
As discussed earlier, much of standard tap water is acidic and may contain other impurities as well. Therefore, a crucial step in regaining and keeping your good health is to drink alkalized water, either by installing a special alkalizing filter in your home or purchasing alkalized water.
Get Off The Couch
As your body moves toward the correct pH balance, you will notice your energy levels will skyrocket! So why not take advantage of this by getting and staying active?
Finding the correct exercise and fitness routine that fits your lifestyle and personal circumstance will take some research and experimenting. However, fitness knowledge is widely available through gym membership, personal trainers, videos, magazines, and the Internet.
The key is to finally get off that couch. Take that first step – get moving, start slowly and go from there.
We Put it All Together
 Make no mistake about it. Our clinic has decades of experience in all aspects of hormone replacement therapy (HRT). We take great pride in the thousands of satisfied clients we have helped, and we are second to none in HRT. We are up-to-date with any-and-all new developments and research discoveries in this ever-evolving area.
Make no mistake about it. Our clinic has decades of experience in all aspects of hormone replacement therapy (HRT). We take great pride in the thousands of satisfied clients we have helped, and we are second to none in HRT. We are up-to-date with any-and-all new developments and research discoveries in this ever-evolving area.
But we go beyond hormone replacement therapy. We leave no stone unturned in addressing all aspects of your good health. That includes lifestyle recommendations that are geared to steer you clear of the many health traps that lie in wait...including your precious and often overlooked acid-alkaline balance!
References
Aihara, Herman (1986) Acid & Alkaline, Orville, CA George Oshawa Macrobiotic Foundation
Johnson, Ben (2011), Healing Waters, Square One Publishers
McCauley, Bob, (2006), The Miraculous Properties of Ionized Water, Spartan Enterprises
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Obese Patients Have a Higher COVID-19 Mortality Risk Than the General Public [Last Updated On: January 24th, 2025] [Originally Added On: August 21st, 2020]
- The Health and Hormone Balancing Qualities of Broccoli [Last Updated On: August 11th, 2025] [Originally Added On: August 27th, 2020]
- What to eat to boost testosterone [Last Updated On: September 14th, 2025] [Originally Added On: December 14th, 2020]
- Breaking a Weight Loss Plateau: How to Reduce Body Fat When Nothing Seems to be Working [Last Updated On: January 20th, 2025] [Originally Added On: February 16th, 2021]
- The Top 25 Most Nourishing and Sustaining Foods to Add to Your Diet Today for Increased Longevity [Last Updated On: January 16th, 2025] [Originally Added On: February 16th, 2021]
- Fight Inflammation and Osteoporosis with Beets! [Last Updated On: January 14th, 2025] [Originally Added On: February 18th, 2021]
- Break a Weight Loss Plateau with Apple Cider Vinegar [Last Updated On: January 14th, 2025] [Originally Added On: February 20th, 2021]
- An Intriguing Look into How Growth Hormone Production and Fasting are Linked [Last Updated On: January 18th, 2025] [Originally Added On: February 21st, 2021]
- Health Reasons for a Vegan Diet [Last Updated On: October 25th, 2025] [Originally Added On: April 2nd, 2021]
- Leafy Greens are Medicine for Your Gut [Last Updated On: September 27th, 2025] [Originally Added On: April 23rd, 2021]
- All Praise to the Spud -- the Delicious, Health-Giving Potato, That Is [Last Updated On: August 12th, 2025] [Originally Added On: June 1st, 2021]
- 16 Cancer-Causing Foods to Avoid [Last Updated On: May 21st, 2025] [Originally Added On: August 12th, 2021]
- Longevity and Anti-Aging -- The Use of Flax Seed Oil [Last Updated On: May 7th, 2025] [Originally Added On: August 17th, 2021]
- Essential Amino Acids Critical to Health and Hormone Balance [Last Updated On: June 13th, 2025] [Originally Added On: October 6th, 2021]
- Growth Hormone and Calcium [Last Updated On: January 11th, 2025] [Originally Added On: October 16th, 2021]
- Growth Hormone and Coffee [Last Updated On: January 9th, 2025] [Originally Added On: October 19th, 2021]
- The Importance of Protein in Weight Loss and Testosterone Production [Last Updated On: January 19th, 2025] [Originally Added On: October 19th, 2021]
- Testosterone, Growth Hormone, and Sugar. [Last Updated On: January 9th, 2025] [Originally Added On: October 19th, 2021]
- Testosterone, Growth Hormone, and Processed Meat [Last Updated On: January 8th, 2025] [Originally Added On: October 19th, 2021]
- Growth Hormone and Intermittent Fasting [Last Updated On: January 7th, 2025] [Originally Added On: October 19th, 2021]
- Growth Hormone and the Importance of Nutrition [Last Updated On: January 7th, 2025] [Originally Added On: October 20th, 2021]
- Growth Hormone Stops Inflammation! [Last Updated On: January 8th, 2025] [Originally Added On: October 20th, 2021]
- Growth Hormone and Sugar Addiction [Last Updated On: February 1st, 2026] [Originally Added On: October 20th, 2021]
- Growth Hormone and Red Meat [Last Updated On: February 3rd, 2026] [Originally Added On: October 20th, 2021]
- Boost Growth Hormone with Sleep [Last Updated On: May 20th, 2025] [Originally Added On: October 20th, 2021]
- A Natural Acid Found in Apples Prevents Muscle Loss AKA Sarcopenia [Last Updated On: January 10th, 2025] [Originally Added On: October 20th, 2021]
- Growth Hormone and Organic Foods. [Last Updated On: January 30th, 2026] [Originally Added On: October 21st, 2021]
- Growth Hormone Food Choices [Last Updated On: January 29th, 2026] [Originally Added On: October 21st, 2021]
- Growth Hormone and Cholesterol: the Surprising Link [Last Updated On: January 6th, 2025] [Originally Added On: October 22nd, 2021]
- Growth Hormone and Weight Loss [Last Updated On: January 31st, 2026] [Originally Added On: October 22nd, 2021]
- Growth Hormone Reduces Inflammation [Last Updated On: February 2nd, 2026] [Originally Added On: October 24th, 2021]
- What You Eat Impacts Both Your Sleep AND Your Growth Hormone Production [Last Updated On: January 10th, 2025] [Originally Added On: October 24th, 2021]
- Growth Hormone Lowers Blood Sugar [Last Updated On: January 6th, 2025] [Originally Added On: October 24th, 2021]
- Testosterone and Magnesium [Last Updated On: May 22nd, 2025] [Originally Added On: April 28th, 2022]
- The Beneficial Functions of Brown Fat vs. White Fat [Last Updated On: May 26th, 2025] [Originally Added On: May 3rd, 2022]
- MOTS-c Peptide for Weight Loss and Muscle Building [Last Updated On: June 11th, 2025] [Originally Added On: November 7th, 2022]
- Looking to lose weight? Add this ingredient to meals [Last Updated On: September 24th, 2025] [Originally Added On: December 7th, 2022]
- The many health benefits of black tea [Last Updated On: September 23rd, 2025] [Originally Added On: December 13th, 2022]
- Raw Food Benefits [Last Updated On: September 20th, 2025] [Originally Added On: January 18th, 2023]
- Brain Foods that Work Together with HGH to Improve Mental Sharpness [Last Updated On: February 14th, 2025] [Originally Added On: March 9th, 2024]