Introduction
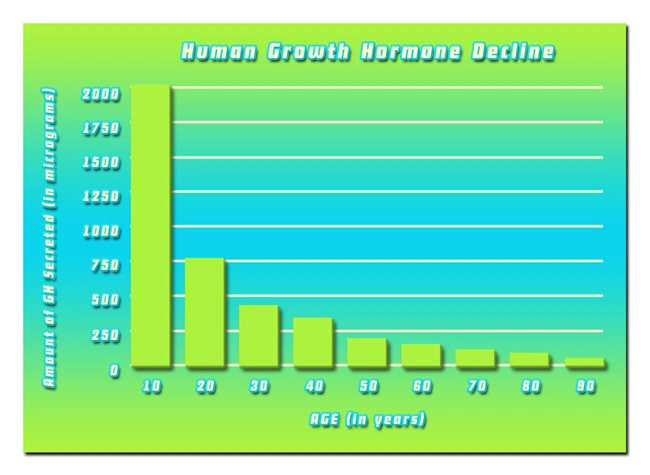
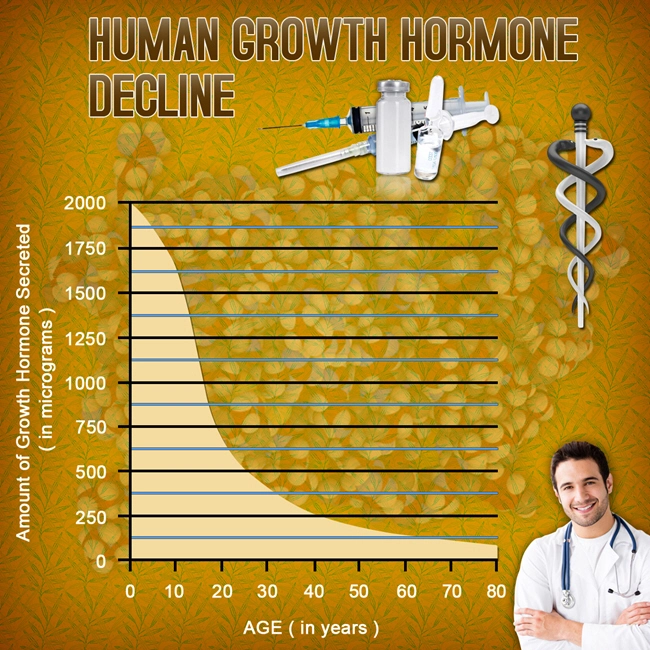
Testosterone replacement therapy (TRT) has become increasingly prevalent among American males seeking to address symptoms associated with hypogonadism. Androgel, a popular topical testosterone gel, has been extensively used for its ease of application and efficacy in boosting testosterone levels. However, the safety and tolerability of Androgel in individuals with pre-existing medical conditions remain a critical area of investigation. This article delves into a Phase IV study aimed at evaluating the safety profile of Androgel in American males with comorbidities, providing valuable insights for healthcare professionals and patients alike.
Study Design and Methodology
The Phase IV study was a prospective, multicenter, observational trial designed to assess the safety and tolerability of Androgel in American males aged 18 to 75 years with hypogonadism and pre-existing medical conditions. Participants were recruited from various clinical settings across the United States, ensuring a diverse sample reflective of the broader population. The study included individuals with conditions such as hypertension, diabetes, cardiovascular disease, and obesity, which are common comorbidities among American males.
Participants were prescribed Androgel according to standard clinical guidelines, and their health was monitored over a 12-month period. Safety assessments included regular blood tests to monitor testosterone levels, hematocrit, and lipid profiles, as well as periodic evaluations of vital signs and adverse events. Patient-reported outcomes were also collected to gauge tolerability and quality of life.
Results: Safety and Tolerability Outcomes
The study found that Androgel was generally well-tolerated among the participants. The incidence of adverse events was comparable to those reported in previous studies of testosterone replacement therapy. Common side effects included skin irritation at the application site, mild fluctuations in mood, and occasional headaches. However, these were mostly transient and did not necessitate discontinuation of therapy.
Importantly, the study did not find a significant increase in cardiovascular events among participants with pre-existing cardiovascular disease. This finding aligns with recent research suggesting that TRT may not pose a substantial risk to cardiovascular health when used appropriately. Similarly, no significant worsening of glycemic control was observed in participants with diabetes, indicating that Androgel can be safely used in this population.
Impact on Comorbidities and Quality of Life
Beyond safety and tolerability, the study also explored the impact of Androgel on participants' comorbidities and overall quality of life. Participants reported improvements in energy levels, mood, and sexual function, which are key indicators of successful TRT. Additionally, there was a modest but significant reduction in body fat percentage among participants with obesity, suggesting that Androgel may offer metabolic benefits.
For individuals with hypertension, the study found no significant changes in blood pressure, indicating that Androgel does not adversely affect this parameter. This finding is crucial for clinicians when considering TRT for patients with hypertension, as it supports the safe use of Androgel in this population.
Clinical Implications and Future Directions
The findings of this Phase IV study provide reassurance regarding the safety and tolerability of Androgel in American males with pre-existing medical conditions. Clinicians can confidently prescribe Androgel to patients with hypogonadism and comorbidities, knowing that the benefits often outweigh the risks. However, ongoing monitoring and individualized treatment plans remain essential to optimize outcomes and minimize potential adverse effects.
Future research should continue to explore the long-term effects of TRT on various health outcomes, including cardiovascular health and metabolic parameters. Additionally, studies examining the impact of different formulations and dosages of testosterone gels could further refine treatment strategies for American males with hypogonadism.
Conclusion
This Phase IV study underscores the safety and tolerability of Androgel testosterone gel in American males with pre-existing medical conditions. By providing robust data on the use of Androgel in diverse patient populations, this research contributes to the growing body of evidence supporting the safe and effective use of TRT. As the prevalence of hypogonadism continues to rise, these findings are invaluable for guiding clinical practice and improving patient care.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Androgel's Impact on Skin Health and Appearance in American Men: Benefits and Risks [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Androgel: A Promising Solution for Male Infertility in American Men [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Androgel Therapy: Economic Impact and Accessibility for American Men with Hypogonadism [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Androgel Therapy: Benefits, Misconceptions, and Informed Decision-Making for American Men [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Androgel: Enhancing Cognitive Function in American Men with Low Testosterone [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Androgel: Enhancing Sleep Quality in American Men with Low Testosterone [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Androgel: Enhancing Life Quality for American Men with HIV/AIDS Through Testosterone Therapy [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Androgel Use and Prostate Health: Risks, Monitoring, and Management Strategies [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Androgel: A Promising Solution for Chronic Pain in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Androgel Use and Hair Loss: Risks, Studies, and Management Strategies [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Androgel: Enhancing Weight Management in Men with Low Testosterone [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Androgel: Enhancing Emotional Well-being in American Men through Testosterone Therapy [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Androgel: Enhancing Vitality in Aging American Men Through Testosterone Therapy [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Androgel's Impact on Kidney Function in American Men: Benefits and Risks [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Androgel: Enhancing Sports Injury Recovery in American Men - Benefits and Risks [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Androgel: A Promising Treatment for Sleep Apnea in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Androgel's Role in Managing Fibromyalgia Symptoms in American Men: A Comprehensive Overview [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Androgel's Impact on Digestive Health in American Men: A Comprehensive Overview [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Androgel's Role in Managing Multiple Sclerosis Symptoms in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Androgel: Balancing Benefits and Heart Health Risks in American Men's Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Androgel's Potential Benefits for Asthma Management in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Androgel: Enhancing Quality of Life for Men on Chemotherapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: A Comprehensive Aid for American Men in Obesity Battle [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: Enhancing Immune Function in American Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: A Promising Treatment for Chronic Fatigue Syndrome in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: A Promising Treatment for Chronic Sinusitis in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: A Potential New Treatment for ADHD in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel: Potential Relief for Seasonal Allergies in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel Use and Potential Hearing Loss in American Men: A Comprehensive Overview [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Maximizing Androgel Therapy Benefits with Lifestyle Changes for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Androgel Use and Hypertension Management in American Men: A Comprehensive Guide [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Androgel Use and Increased Blood Clot Risk in American Men: A Comprehensive Overview [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Androgel Use and Its Effects on Thyroid Health in Men: A Comprehensive Guide [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Androgel's Potential Benefits for American Men with Epilepsy: A Comprehensive Overview [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Androgel's Potential in Managing Migraines: A Review for American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Androgel: A Promising Therapy for CKD and Low Testosterone in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel Use and Liver Health: Monitoring and Lifestyle Tips for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel: A Novel Approach to Managing Arthritis Pain in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel: Exploring Its Potential in Preventing Eye Diseases in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel Use and Its Impact on Dental Health in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel: Benefits for Low Testosterone vs. Potential Skin Cancer Risks in Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel: Enhancing Life Quality in Men with Autoimmune Disorders [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel's Potential Benefits for American Men with ALS: A Comprehensive Overview [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel's Potential in Managing Parkinson’s Disease in American Men: Current Insights [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Androgel's Role in Enhancing Stroke Recovery: Benefits and Safety Considerations [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Androgel: Enhancing Post-Surgical Recovery in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Androgel: Potential Benefits and Cautions for Anxiety Management in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Androgel: Enhancing Life Quality for American Men with COPD Through Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Androgel Use in Diabetic Men: Benefits, Risks, and Management Strategies [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Androgel's Role in Managing Gout: Benefits and Clinical Insights for American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Androgel Use in Men with Crohn's: Management Strategies and Considerations [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Androgel's Potential in Managing Rheumatoid Arthritis for American Men: A Review [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Androgel: A Promising Therapy for Scleroderma in American Men [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Androgel's Potential in Managing Ulcerative Colitis in American Men: An Overview [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Androgel's Potential in Managing Lupus Symptoms in American Men: A Comprehensive Review [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Navigating Androgel Use and Celiac Disease: A Comprehensive Guide for American Men [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Androgel's Role in Enhancing Heart Attack Recovery in American Men [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Androgel: A Novel Adjunct Therapy for Psoriasis in American Men [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Androgel Use in American Men: Managing Acne Side Effects Effectively [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Androgel: A Potential but Unproven Treatment for Eczema in American Men [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Androgel: A Promising Treatment for Alopecia in American Men [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Androgel's Role in Managing Rosacea: Efficacy and Application in American Men [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Androgel Use and Shingles Management: A Comprehensive Guide for American Men [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Androgel: A Promising Aid for American Men Managing Herpes Outbreaks [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Androgel: Enhancing Life Quality for American Men with HIV through Testosterone Therapy [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Androgel's Potential in Managing Vitiligo for American Men: A Comprehensive Overview [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Androgel Enhances Burn Recovery in American Men: Physical and Psychological Benefits [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Androgel's Potential Role in Malaria Treatment: A Promising Yet Unproven Hypothesis [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Androgel's Potential in Managing Zika Symptoms in American Men: A Testosterone Therapy Insight [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Androgel's Role in Managing Chikungunya Symptoms in American Men [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Androgel's Potential in Enhancing Hepatitis C Treatment for American Men [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Androgel's Potential in Enhancing Immune Response to Ebola in American Men [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Androgel's Role in Enhancing Dengue Recovery Among American Males [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Lyme Disease in American Men: Androgel's Role in Managing Symptoms [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Androgel's Potential in Treating Tuberculosis Among American Men: A New Approach [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Exploring Androgel's Potential in Managing Yellow Fever in American Men [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Androgel Use and West Nile Virus: Impacts on American Men's Health [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Androgel's Potential in Managing Rabies Symptoms: A Neuroprotective Approach [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Androgel's Impact on Flu Recovery in American Men: Benefits and Precautions [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Androgel: Comprehensive Guide to Testosterone Therapy for American Males with Hypogonadism [Last Updated On: April 23rd, 2025] [Originally Added On: April 23rd, 2025]