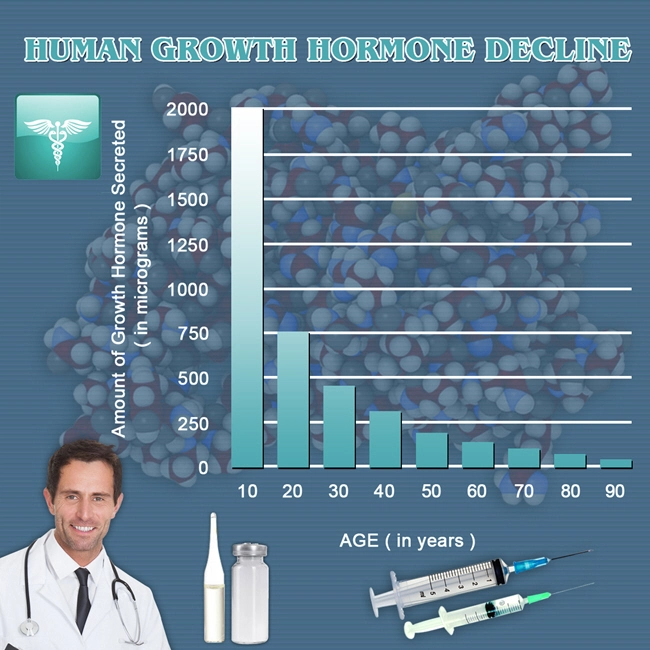
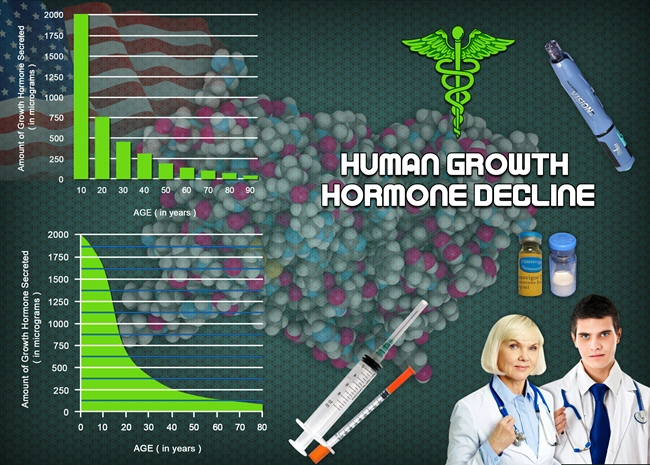
Description
Nutropin is a Growth Hormone derived from recombinant DNA. Nutropin is of 191residue amino acids and has a molecular weight of 22,125 daltons. The sequence of Nutropin's amino acids is perfectly identical to the sequence of HGH derived naturally from the pituitary. Nutropin is from a specific lab strain of the E. coli bacteria as a precursor chemical. It consists of the recombinant Human Growth Hormone molecule that is by a signal for secretion that originates with a protein from the E. coli bacteria.
This precursor chemical travels through the protective membranes of the cells. After the E. Coli secretion signal is initiated, the native protein enters into the  periplasm, and the protein begins to be folded correctly into Human Growth Hormone.
periplasm, and the protein begins to be folded correctly into Human Growth Hormone.
Nutropin is an intensely purified preparation. The biological potency of Nutropin is configured through a bioassay designed to proliferate Growth Hormone cells.
Nutropin is a white, sterile freeze-dried powder that is by subcutaneous injection after it has with Bacteriostatic Water for Injection, USP, and the preservative benzyl alcohol. After the product has been reconstituted, it is nearly isotonic at a 5 mg/mL concentration of Human Growth Hormone. It also has a pH of about 7.4.
Every 5 mg vial of Nutropin holds 5 mg (about 15 IU) somatropin which has 1.7mg glycine 45 mg of mannitol, and 1.7 mg sodium phosphates (1.3 mg sodium phosphates dibasic, 0.4 mg sodium phosphate monobasic).
Every 10 mg vial of Nutropin holds 10 mg (about 30 IU) somatropin which has 3.4mg glycine 90 mg of mannitol, and 3.4 mg sodium phosphates (2.6 mg sodium phosphates dibasic, 0.8 mg sodium phosphate monobasic).
Bacteriostatic Water for Injection, USP, is sterilized water that contains 0.9% benzyl alcohol per milliliter. This sterilized water is in a vial that can be used multiple times. The benzyl alcohol acts as a preservative that protects the fluid from microbial contamination. The pH of the dilutant is 4.5-7.
Clinical Pharmacology
General
In vivo and in vitro clinical and preclinical testing has provided ample evidence that Nutropin is equivalent therapeutically to Human Growth Hormone naturally derived from the pituitary. Children who do not secrete adequate levels of Growth Hormone endogenously, patients who suffer from chronic renal insufficiency (CRI), and patients who suffer from Turner syndrome all report benefits when taking Nutropin.
Patients who took Nutropin were found to experience an increase in their rate of growth and also an increase in their Insulin-like Growth Factor-1 (IGF-I) levels in a manner that is compatible with Human Growth Hormone naturally produced by the pituitary.
Benefits that have been shown through studies regarding Nutropin, somatrem, somatropin, and/or Human Growth Hormone derived naturally from the pituitary include:
A.Tissue Growth --
1) Skeletal Growth: Growth Hormone stimulates skeletal growth in
young patients that experience growth failure as a result of inadequate secretion of natural Growth Hormone, or as a secondary effect of chronic kidney insufficiency, or also with patients that suffer from Turner syndrome. Turner syndrome is a genetic disorder that seriously hinders hormone production and has devastating effects on the physiology of a woman from birth. It is caused when the second chromosome does not form properly or does not develop at all, leaving only one functional X chromosome.
Growth of the long bones of the skeleton is a result of the expansion of the epiphyseal plates that are on the ends of the growing bone. The metabolism and growth of the cells of the epiphyseal plates are stimulated directly by Growth Hormone and Insulin-like Growth Factor-1, an important stimulator of Growth Hormone. Levels of Insulin-like Growth Factor-1 in the blood serum are low in adolescents and children who are deficient in GH, but their levels increase when treated with Growth Hormone.
In these young patients, the new bone begins to develop in the growth plates after Growth Hormone and Insulin-like Growth Factor-1 have been introduced to the body. As one reaches the end of puberty, the growth plates fuse, and adult height is in.
2) Cell Growth: Human Growth Hormone leads to an increase in both the size and number of skeletal muscle cells. This leads to enhanced strength as there are more muscle fibers. Each individual muscle fiber can handle a larger load as well.
3) Organ Growth: Growth Hormone has a direct effect on the size of the internal organs, including the kidneys. It also increases the mass of red cells. Growth Hormone Treatment of genetic dwarf or hypophysectomized rats (rats that had their pituitary glands removed) leads to organ development that is in proportion  to the growth of the rest of the body. In healthy rats that were induced with uremia (high levels of nitrogen) through nephrectomy, Growth Hormone promoted body and skeletal growth.
to the growth of the rest of the body. In healthy rats that were induced with uremia (high levels of nitrogen) through nephrectomy, Growth Hormone promoted body and skeletal growth.
B. Protein Metabolism: Proper growth is in part by protein synthesis which is caused by Growth Hormone. This is through retention of nitrogen that reveals itself through a lower level of nitrogen excreted through the urine, as well as clinical testing for nitrogen in the blood urea which occurs during Growth Hormone Therapy.
C. Carbohydrate Metabolism: Growth Hormone is a significant controlling mechanism in charge of carbohydrates. In patients that do not adequately secrete Growth Hormone, hypoglycemia is during periods of fast. This issue is largely resolved with Human Growth Hormone Therapy. It is a possibility that Growth Hormone therapy can lead to a decrease in the body's sensitivity to insulin.
Patients who are not for chronic kidney insufficiency and/or Turner syndrome have a higher risk to develop intolerance to glucose. Human Growth Hormone treatment provided to children or adults has resulted in an increase in post-meal insulin levels and serum fasting. These two results happen most commonly with those that are overweight or obese. Also, post-meal glucose, mean fasting, and A1c hemoglobin (a measure of blood glucose) stay at a healthy level.
D. Lipid Metabolism: In patients who are Growth Hormone Deficient, Growth Hormone Therapy led to the mobilization of lipids, a reduction in stores of body fat, increased fatty acids entering the plasma, and lower levels of cholesterol in the plasma.
E. Mineral Metabolism: Use of Growth Hormone leads to the retention of potassium in the body. This retention apparently is due to the enhanced cell growth that begins to occur. Inorganic phosphorus levels in the blood serum can sometimes increase modestly among patients who undergo Growth Hormone Therapy because they do not produce enough Growth Hormone on their own, have a chronic kidney deficiency, or suffer from Turner syndrome.
This is because Growth Hormone enhances the metabolic processes associated with the growth of bone, in addition to improving tubular reuptake of phosphate by the kidneys. Serum calcium levels do not change significantly among these patients. Retention of sodium also occurs. Adults that developed a Growth Hormone Deficiency as children display low levels of bone mineral density (Acronym: BMD). (See also Precautions: Laboratory Tests.)
F. Metabolism of the Connective Tissues: Growth Hormone chondroitin sulfate synthesis and collagen synthesis in addition to encouraging the urinary expulsion of hydroxyproline.
Pharmacokinetics
Absorption after Subcutaneous Injection The total bioavailability of recombinant HGH after being injected subcutaneously into a healthy adult male is to be 81 plus or minus20%. The terminal mean t after subcutaneous injection is much longer than the terminal mean after intravenous injection (2.1 plus or minus 0.43 hr vs. 19.5 plus or minus 3.1m
in). This indicates that subcutaneous injection of Growth Hormone causes the compound to be released slowly and at a more modest rate.
Distribution Recombinant Human Growth Hormone Animal Research has shown that Growth Hormone is vital to organ function, especially to the kidneys and liver. The distribution volume at steady state for Recombinant Human Growth Hormone in a healthy adult male is around 50 mL/kg of body weight, essentially the serum volume.
Metabolism Both the kidney and liver have been proven to be essential organs for the proper metabolism of Growth Hormone. Animal research provides evidence that the kidneys are the primary organs of clearance. Growth Hormone filtration occurs at the glomerulus and Growth Hormone is by the proximal tubules. After that, it is inside the kidney cells into its component amino acids, after which the acids return to normal circulation.
Elimination--The terminal mean t after intravenous injection of recombinant Human Growth Hormone adult males in good health is to be 19.5 plus or minus 3.1 minutes. Elimination of recombinant Human Growth Hormone after intravenous injection among healthy children and adults is to range from 116-174 mL/hr/kg.
Formulation bioequivalence Nutropin [somatropin injection derived from recombinant DNA] has to be bioequivalent of Nutropin [somatropin injection derived from recombinant DNA]. This is upon statistical evaluation of Area Under the Curve (AUC) and mean C.
Special Populations
Pediatric Current research data suggests that there is no difference in Human Growth Hormone clearance between children and adults.
Gender There is no data available for exogenously injected recombinant Human Growth Hormone. The data that has been collected regarding methionyl Human Growth Hormone naturally produced and extracted pituitary Growth Hormone, and endogenous Growth Hormone produces no evidence that suggests that men and women clear GH at different rates.
Geriatrics The limited amount of published research shows that Growth Hormone plasma clearance and normal steady-state GH plasma concentration do not appear to be different among young and elderly populations.
Race The half-life values for endogenous Growth Hormone in healthy black males are not to be any different than the half-life values of Growth Hormone for healthy white males. There are no available data regarding other races.
Growth Hormone Deficiency (GHD) The reported values regarding the clearance of recombinant Human Growth Hormone in children and adults suffering from GHD range from 138-245 mL/hr/kg. These values are similar to those that occur in healthy children and adults. The terminal mean t values following subcutaneous and intravenous administration in pediatric and adult Growth Hormone Deficiency patients have also proven to be similar to the values observed in the population of healthy adult males.
Kidney Insufficiency Adults and children who suffer from end-stage renal disease (ESRD) and chronic renal failure (CRF) clear Human Growth Hormone at a  lower rate than healthy individuals. Endogenous Growth Hormone production can sometimes increase in individuals suffering from ESRD. Even so, no accumulation of Human Growth Hormone has in children with ESRD of CRF that are normal Growth Hormone Replacement Therapy.
lower rate than healthy individuals. Endogenous Growth Hormone production can sometimes increase in individuals suffering from ESRD. Even so, no accumulation of Human Growth Hormone has in children with ESRD of CRF that are normal Growth Hormone Replacement Therapy.
Turner Syndrome No pharmacokinetic data is currently available regarding Turner syndrome and Growth Hormone Therapy. It has, however, that absorption, half-life, and clearance rates for internally produced Growth Hormone for this population show little difference when compared to the rates of healthy patients and patients only suffering from Growth Hormone Deficiency.
Hepatic Insufficiency A reduction in recombinant Growth Hormone clearance has in patients that are suffering from extreme liver dysfunction. The clinical significance of this data is still unknown.
Summary of Pharmacokinetic Nurotropin Parameters in Adult Men of Good Health
0.1 mg(about 0.3 IU a)/kg SubC max C
(g/L) max T
(hour) t1/2
(hour)(Area Under the Curve)
ghr/L) Ev/F SubC
(mL/[hrkg])
Averageb
67.2 / 6.2/ 2.1 / 643 / 158
CV
29 / 37 /20 / 12 / 12
Key
maximum
t1/2life
Ev=systemic
F SubC=biological subcutaneously (indeterminant);
CV=variation coefficient
SubC=subcutaneous
a
b
Effectiveness Studies
Nutropin's upon as an of CRI
Two randomized, controlled, multi-center clinical trials were conducted in an effort to learn whether Nutropin treatment before patients who are suffering from chronic renal insufficiency receive renal transplants could possibly lead to an improvement in growth rate and a decrease in height deficit. One of the studies was  a placebo-controlled, double-blind trial, and the second trial was randomized and open-label. In both of these controlled studies,that participants took a Nutropin dose of 0.05 mg/kg/day (0.35mg/kg/wk). These doses were given daily by means of subcutaneous injection.
a placebo-controlled, double-blind trial, and the second trial was randomized and open-label. In both of these controlled studies,that participants took a Nutropin dose of 0.05 mg/kg/day (0.35mg/kg/wk). These doses were given daily by means of subcutaneous injection.
Taking data from both studies of patients who underwent treatment for two full years resulted in 62 participants who received Nutropin treatment and 28 participants who were either untreated or treated by placebo. The year one mean rate of growth was 10.8 cm/yr for patients treated with Nutropin, as compared to an average rate of growth of 6.5 cm/yr for the untreated/placebo controls.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Injectable HGH Prescriptions: Blood Testing Centers in Philadelphia, Pennsylvania [Last Updated On: August 27th, 2025] [Originally Added On: March 3rd, 2019]
- Nutropin Hgh: Hgh Hormone Product [Last Updated On: December 21st, 2024] [Originally Added On: March 10th, 2020]
- Hgh Replacement [Last Updated On: December 21st, 2024] [Originally Added On: March 14th, 2020]
- Hgh Therapy [Last Updated On: December 22nd, 2024] [Originally Added On: March 15th, 2020]
- Anti-ageing Clinics Distribute Human Growth Hormone [Last Updated On: December 25th, 2024] [Originally Added On: March 16th, 2020]
- Hgh In Forefront To Remain Young [Last Updated On: November 25th, 2024] [Originally Added On: March 18th, 2020]
- Benefits Of Hgh: Thierry Hertoghe [Last Updated On: December 23rd, 2024] [Originally Added On: April 23rd, 2020]
- Getting Started With An Hgh Program [Last Updated On: December 24th, 2024] [Originally Added On: April 25th, 2020]
- Hgh Releasers [Last Updated On: December 25th, 2024] [Originally Added On: April 26th, 2020]
- What dietary considerations should I make while on HRT, hormone replacement therapy? [Last Updated On: December 20th, 2024] [Originally Added On: May 1st, 2020]
- Hgh Dosage [Last Updated On: March 3rd, 2025] [Originally Added On: May 5th, 2020]
- Hgh Deficiency [Last Updated On: December 23rd, 2024] [Originally Added On: May 6th, 2020]
- Hgh Products Genotropin Humantrope Nutropin Saizen Serostim Omnitrope [Last Updated On: November 25th, 2024] [Originally Added On: May 8th, 2020]
- What Is Injectable Hgh Human Growth Hormone? [Last Updated On: December 22nd, 2024] [Originally Added On: May 9th, 2020]
- Introduction To Human Growth Hormone HGH [Last Updated On: December 24th, 2024] [Originally Added On: May 10th, 2020]
- Omnitrope Injections for the Treatment of HGH Deficiency [Last Updated On: June 15th, 2025] [Originally Added On: May 6th, 2021]
- Sleep To Amplify Human Growth Hormone Levels [Last Updated On: June 14th, 2025] [Originally Added On: May 27th, 2021]
- Buying Hgh Hormone Human Growth Hormone [Last Updated On: August 20th, 2025] [Originally Added On: February 22nd, 2022]
- Hgh For Menopause [Last Updated On: October 28th, 2025] [Originally Added On: February 23rd, 2022]
- Your HGH Levels [Last Updated On: October 5th, 2025] [Originally Added On: February 24th, 2022]
- Related Glossary of Terms [Last Updated On: October 14th, 2024] [Originally Added On: February 25th, 2022]
- Hgh Facts - Information About Growth Hormone [Last Updated On: August 21st, 2025] [Originally Added On: February 26th, 2022]
- Hgh Sports And Baseball [Last Updated On: August 22nd, 2025] [Originally Added On: February 27th, 2022]
- Us Olympic Committee And Talk Of Hgh [Last Updated On: October 30th, 2025] [Originally Added On: February 28th, 2022]
- Braves' Schafer Talks About His Suspension Over Hgh [Last Updated On: August 23rd, 2025] [Originally Added On: March 1st, 2022]
- Hgh Scams: Different Hgh Scams And Things To Watch For Online [Last Updated On: August 27th, 2025] [Originally Added On: March 2nd, 2022]
- Nordiflex HGH Injection Device Information [Last Updated On: October 31st, 2025] [Originally Added On: March 3rd, 2022]
- Genotropin Hgh Detailed Description [Last Updated On: October 15th, 2024] [Originally Added On: March 4th, 2022]
- Saizen Hgh Detailed Description [Last Updated On: October 17th, 2024] [Originally Added On: March 6th, 2022]
- Genotropin HGH: Human Growth Hormone Product [Last Updated On: August 24th, 2025] [Originally Added On: March 6th, 2022]
- Omnitrope Hgh: Human Growth Hormone Product Called Omnitrope. [Last Updated On: October 5th, 2024] [Originally Added On: March 7th, 2022]
- Saizen Hgh: Injectable Hgh Product [Last Updated On: August 25th, 2025] [Originally Added On: March 9th, 2022]
- Hgh Product Serostim [Last Updated On: August 26th, 2025] [Originally Added On: March 11th, 2022]
- HGH Scams (HGH Supplements) [Last Updated On: November 12th, 2024] [Originally Added On: March 20th, 2022]
- Side Effects Of HGH [Last Updated On: September 3rd, 2025] [Originally Added On: March 21st, 2022]
- Testimonial: Walter’s HGH Story [Last Updated On: September 28th, 2025] [Originally Added On: June 4th, 2022]
- HGH Is the “God Particle” [Last Updated On: September 17th, 2025] [Originally Added On: July 26th, 2022]
- Low testosterone in men might increase the risk of severe COVID [Last Updated On: October 21st, 2025] [Originally Added On: September 6th, 2022]
- Can I Reverse the Aging Process with HGH? [Last Updated On: September 19th, 2025] [Originally Added On: September 21st, 2022]
- A new study confirms: Testosterone Replacement Therapy is safe! [Last Updated On: October 4th, 2025] [Originally Added On: October 2nd, 2022]
- HGH Therapy – How Can I Know Who Is Legitimate? [Last Updated On: June 7th, 2025] [Originally Added On: January 10th, 2023]
- The Evolution of Testosterone Replacement Therapy for men with Prostate Cancer [Last Updated On: June 2nd, 2025] [Originally Added On: January 12th, 2023]
- Nine Hormones that Can Impact Your Weight and How to Promote Hormone Balance [Last Updated On: June 6th, 2025] [Originally Added On: January 26th, 2023]
- Get Tested for Hormone Levels, even if You Have No Symptoms [Last Updated On: September 29th, 2025] [Originally Added On: March 17th, 2023]
- Can Head Injuries Cause HGH Deficiency? [Last Updated On: September 26th, 2025] [Originally Added On: May 17th, 2023]
- Mountain Biker Thought his Life Was Over [Last Updated On: September 15th, 2025] [Originally Added On: May 25th, 2023]
- Do HGH Booster Supplements Work Faster Than Prescriptions? [Last Updated On: October 1st, 2025] [Originally Added On: June 3rd, 2023]
- HGH and It’s Effects on Other Hormones [Last Updated On: October 26th, 2024] [Originally Added On: June 10th, 2023]
- Maintaining a Proper Medication Schedule [Last Updated On: November 24th, 2024] [Originally Added On: August 23rd, 2023]
- What is the History of the Discovery of HGH? [Last Updated On: October 21st, 2024] [Originally Added On: September 17th, 2023]
- Don’t Let Fear Stop You From Fixing Your Hormones [Last Updated On: October 22nd, 2024] [Originally Added On: September 22nd, 2023]
- Understanding HGH Dosage [Last Updated On: February 26th, 2025] [Originally Added On: February 26th, 2025]