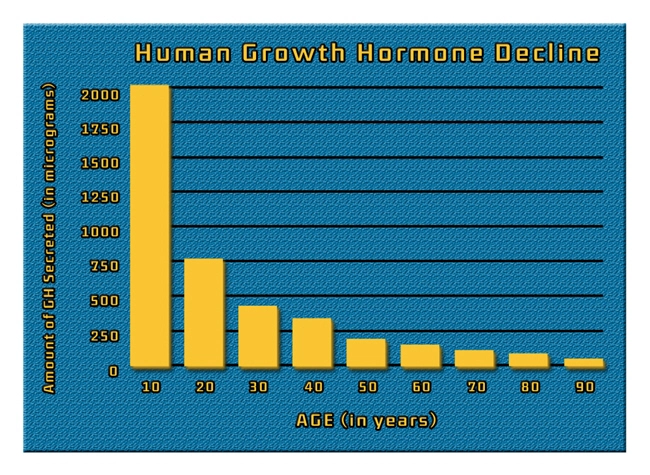
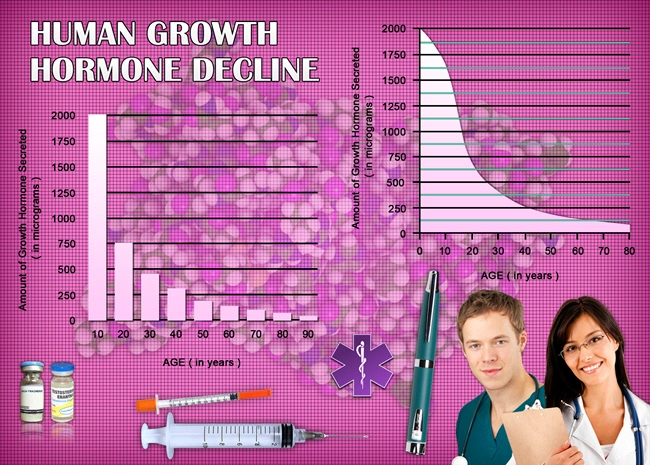
Description:
Lyophilized Powdered Genotropin contains Recombinant-DNA [rDNA] derived somatropin. Somatropin is a polypeptide hormone comprised of a chain of 191 residues of amino acid, and it has a molecular weight o 22,124 daltons. Somatropin's sequence of amino acids is exactly the same as that of Human Growth Hormone which originates in the pituitary gland.
Genotropin is created through a modification of a particular strain of E. coli to include the gene that is responsible for Human Growth Hormone. Genotropin comes in the form of a white, sterile powder, lyophilized and intended for injection subcutaneously.
Genotropin 1.5 milligram is administered using a 2-chambered cartridge. The front compartment holds 1.5 milligrams of rDNA-derived somatropin (about 4.5  IU),27.6 milligrams of glycine, 0.3 milligrams or anhydrous sodium dihydrogen phosphate, and 0.3 milligrams of anhydrous disodium phosphate. The rear compartment holds 1.13 milliliters of water used for injection.
IU),27.6 milligrams of glycine, 0.3 milligrams or anhydrous sodium dihydrogen phosphate, and 0.3 milligrams of anhydrous disodium phosphate. The rear compartment holds 1.13 milliliters of water used for injection.
Genotropin 5.8 milligram is administered using a 2-chambered cartridge. The front compartment holds 5.8 milligrams of rDNA-derived somatropin (about 17.4 IU), 2.2 milligrams of glycine, 1.8 milligrams of mannitol, 0.32 milligrams of anhydrous sodium dihydrogen phosphate, and 0.31 milligrams of anhydrous disodium phosphate. The rear compartment holds 0.3 per cent m-Cresol for use as a preservative, a long with 45 milligrams of mannitol and 1.14 milliliters of water to be used for injection.
Genotropin 13.8 milligram is administered using a 2-chambered cartridge. The front compartment holds 13.8 milligrams of rDNA-derived somatropin (about 41.4 IU), 2.3 milligrams of glycine, 14.0 milligrams of mannitol, 0.47 milligrams of anhydrous sodium dihydrogen phosphate, and 0.46 milligrams of anhydrous disodium phosphate. The rear compartment holds 0.3 per cent m-Cresol for use as a preservative, a long with 32 milligrams of mannitol and 1.13 milliliters of water to be used for injection.
Genotropin Miniquick is administered by a one-time use syringe device that holds a 2-chambered cartridge. Genotropin Mini quick can be attained in single doses varying from 0.2 milligrams to 2.0 milligrams in increments of 0.2 milligrams. The front compartment holds 0.22 to 2.2 milligrams of rDNA-derived somatropin (about 0.66 to 6.6 IU), 0.23 milligrams of glycine, 1.14 milligrams of mannitol, 0.05 milligrams of anhydrous sodium dihydrogen phosphate, and 0.027 milligrams of anhydrous disodium phosphate. The rear compartment holds 12.6 milligrams of mannitol in 12.6 milliliters of water to be used for injection 0.275 milliliters.
Genotropin is prepared in a highly purified manner. The recombinant reconstituted solution of somatropin has a pH of about 6.7 and an osmolality of about 300 mOsm/kg. The concentration of the somatropin solution is dependent upon the presentation and strength (read more in the section how Supplied).
Clinical Pharmacology
Clinical, preclinical, and in vitro tests show that Powdered Lyophilized Genotropin is equivalent to human Growth Hormone that originates naturally in the pituitary in terms of therapeutic results.
The body reacts in a similar fashion to Genotropin as it does to the Human Growth Hormone created internally in the average adult. Genotropin therapy in child patients who suffer from a growth hormone deficiency (GHD) succeeds in stimulating normal growth and balances concentrations of Somatomedin-C/Insulin-like Growth Factor (IGF-1). Genotropin treatment in adults who are maligned with Growth Hormone Deficiency often results in a lower level of body fat, an increased amount of lean muscle mass, and changes in metabolism that include positive changes in the metabolism of lipids and the balance of concentrations of IGF-1.
Also, many other results have been shown through the administration of somatropin and/or Genotropin, including:
Tissue Growth
Skeletal System Growth: Genotropin has been shown to stimulate the growth of the skeletal system in young patients who suffer from Growth Hormone Disorder. There is a significant increase in the length of the body through the use of Genotropin that results from its effect upon the epiphyseal plates which develop inside the long bones. Serum levels of Insulin-Like Growth Factor-1, which likely play a part in the growth of the skeletal system, are found to usually be quite low in young patients suffering from Growth Hormone Disorder.
These levels increase when the patient undergoes Growth Hormone Replacement Therapy with Genotropin. Higher serum concentrations levels of alkaline phosphatase also are seen with treatment.
Cell Growth: Research has shown that young, short-statured children have a lesser number of skeletal muscle cells because they have an insufficient level of  natural Growth Hormone in comparison to the normal child population. Treatment with Genotropin leads to a boost in both the size and number of these muscle cells.
natural Growth Hormone in comparison to the normal child population. Treatment with Genotropin leads to a boost in both the size and number of these muscle cells.
Protein Metabolism
Linear, normal growth in childhood is in part facilitated through an increase in the synthesis of cellular proteins. Retention of nitrogen, which is shown through a decrease in the excretion of nitrogen through urination, as well as through blood test measurements of nitrogen in the urine, is one of the many physiological changes that occur through Genotropin therapy.
Carbohydrate Metabolism
Children who suffer from hypopituitarism often also experience hypoglycemia when fasting, and this issue is also alleviated through Genotropin treatment, though excessive doses of Human Growth Hormone can lead to glucose intolerance.
Lipid Metabolism
In those who are deficient in growth hormone, Genotropin therapy has been shown to result in the mobilization of lipids, allowing for a reduction in storage of body fats and a higher level of fatty acid in the plasma.
Mineral Metabolism
Genotropin also can lead to are retention of phosphorus, potassium, and sodium. Concentrations of inorganic phosphate in the blood stream are also increased in those patients maligned with Growth Hormone Disorder who go through Genotropin Hormone Therapy. Levels of calcium in serum are not changed in a significant manner through the use of Genotropin, and Human Growth Hormone can lead to calciuria.
Body Composition
Adult patients who suffer from Growth Hormone Disorder that are treated with Genotropin at the suggested adult dosage (view Dosage and Administration for more information) show a decreased level of body fat and an increased level of lean muscle mass. These changes occur at the same time that the body begins to retain fluid more effectively. The result of all of this is that Genotropin has the ability to positively modify the composition of the body, and these positive changes can be sustained through continuing treatments.
Pharmacokinetics
Absorption
After delivering a 0.03milligram/kilogram subcutaneous (abbreviated SC) thigh injection of 1.3 milligram/milliliter Genotropin to an adult patient with Growth Hormone Disorder, around 80% of the total dose was available for usage by the body in comparison to that available to the body through an intravenous dose. The results were similar in both female and male hormone replacement therapy patients, and a similar level of bioavailability has been shown in healthy male adult subjects as well.
In adult healthy males, after a subcutaneous thigh injection of 0.03 mg/kg, the extent of AUC absorption of a 5.3 mg/ml concentration of Genotropin proved to be 35% higher than that from 1.3 mg/mL Genotropin. The mean (plus or minus standard deviation) peak (max C) serum levels were 23.0 (plus or minus 9.4) ng/mL and 17.4 (plus or minus 9.2 ng/mL, respectively.
In another study of a similar kind with pediatric Growth Hormone Deficiency patients, the dosage of 5.3 mg/mL Genotro
pin produced a mean area under the curve that happened to be 17% higher than the mean AUC for 1.3 mg/mL Genotropin. The mean max C levels were 21.0 ng/mL and 16.3 ng/ml respectively.
In yet another study, Adult GHD sufferers were administered two separate subcutaneous doses of 0.03 mg/kg Genotropin at a 1.3 mg/mL concentration. There was a 1-4 week period to washout between the injections. The mean max C levels were 12.4 ng/mL for the first administration and 12.2 ng/mL for the second injection and this was reached at around 6 hours after the dose was received.
No data exists regarding bioequivalence between the 12 mg/mL dosage and the 1.3 mg/mL or the 5.3 mg/ml doses.
Distribution
The mean distribution volume of Genotropin after it has been administrated to adults with Growth Hormone Deficiency was figured to be about 1.3 (plus or minus 0.8)L/kg.
Metabolism
Genotropin is metabolized through normal protein catabolism which occurs in both the kidneys and the liver. In the kidney cells, some of the products of Genotropin's breakdown are filtered back into the circulation of the system.
 The terminal mean half-life of Genotropin which is administered intravenously in a normal adult is around 24 minutes, but when Genotropin is administered subcutaneously, it has a half-life of 180 minutes in adults with Growth Hormone Disorder. This significant difference in half-life is due to the fact that when Genotropin is administered subcutaneously it absorbs into the body at a much slower rate.
The terminal mean half-life of Genotropin which is administered intravenously in a normal adult is around 24 minutes, but when Genotropin is administered subcutaneously, it has a half-life of 180 minutes in adults with Growth Hormone Disorder. This significant difference in half-life is due to the fact that when Genotropin is administered subcutaneously it absorbs into the body at a much slower rate.
Excretion
The mean of clearance or excretion of Genotropin which has been administered subcutaneously in a study of 16 adult subjects with Growth Hormone Disorder was 0.3 (plus or minus 0.11) L/hrs/kg.
Special Populations
Pediatric: Genotropin is broken down in adults in a similar manner as it is broken down in children.
Gender: There have been no studies that attempt to differentiate the difference in effect that Genotropin has between young boys and young girls, but it should be noted that Genotropin bioavailability has been found to be similar between adult females undergoing Growth Hormone Replacement Therapy and Male Hormone Replacement Therapy patients in regard to Growth Hormone Deficiency.
Race: No research has been conducted with regard to whether race plays a factor in the pharmacokinetic results of Genotropin use.
Hepatic or renal insufficiency: There have been no research studies conducted in regard to Genotropin's effect on these particular populations of patients.
Table One
Average subcutaneous pharmacokinetic parameters of adult patients who are deficient in growth hormone.
Bio-availability
(percentage)
(Number of participants = 15) MaxT
(Time [hours])
(Number of participants = 16) Clearance Rate
(Liters/hour ' kilogram)
(Number of participants = 16)Vol.Dis.
(Liters/kilogram)
(Number of participants = 16) T
(hours)
(Number of participants =16)
Average 80.5 / 5.9 / 0.3 / 1.3 /3.0
(St.Dev.) * ( 1.65) ( 0.11) ( 0.80) ( 1.44)
95% CI 70.5 to 92 /. 5.0 to 6.7 / 0.2 to 0.4 / 0.9 to 1.8 / 2.2 to 3.7
Max T= Time that has elapsed when plasma concentration peaks
Clearance Rate = rate in which Genotropin is excreted from the body
Vol/Dis = distribution volume
T = time that it takes the plasma concentration of Genotropin to reduce by half from its peak
St.Dev. = standard deviation
Cl = interval of confidence
Absolute Genotropin bioavailability has been estimated with the assumption that the data which has been transformed by log will follow a normal, standard distribution. The standard deviation and mean of the transformed were a mean of 0.22 and a standard deviation of plus or minus 0.241.
Clinical research with adult growth hormone Disorder patients
Lyophilized Genotropin Powder was tested alongside a placebo in 6 clinical randomized trials which involved 172 adult Growth Hormone Disorder patients in  total. The trials featured a six-month treatment period that was double-blind in nature. During this period, 85 participants were administered Genotropin and 87 participants were administered a placebo. After this, an open-label period of treatment followed. During this period, patients who chose to participate were administered Genotropin for a period of time as long as 24 months.
total. The trials featured a six-month treatment period that was double-blind in nature. During this period, 85 participants were administered Genotropin and 87 participants were administered a placebo. After this, an open-label period of treatment followed. During this period, patients who chose to participate were administered Genotropin for a period of time as long as 24 months.
Genotropin was injected subcutaneously each day with a dosage of 0.04 mg/kg dose of 0.04 mg/kg/wk during month one of treatment and in the subsequent months thereafter it was administered at 0.08 mg/kg/wk
After the six-month period of treatment, researchers looked for changes that were beneficial to the composition of the body for both the group that received Genotropin injections as well as the group which received injections of placebo.
It was found that in the Genotropin group lean muscle mass, total water retention, and the ratio of lean/fat improved, while the circumference of the waist and total mass of body fat reduced to more healthy levels.
The effects of Genotropin on the composition of the body were retained with those who pursued treatment past the initial six-month period. Mineral bone density began to decline after six months of treatment, but after twelve months of treatment, the density values returned to baseline.
Usage indications
Lyophilized Genotropin Powder is designed for long-term therapy of pediatric patients that suffer from growth failure as a result of the inadequate production of endogenous Human Growth Hormone. It is not recommended for other causes of short stature.
Genotropin is also designed for long-term Growth Hormone Replacement Therapy for adults that suffer from a Growth Hormone Deficiency that is of either adult or childhood-onset in its etiology. Growth Hormone Disorder should be diagnosed through an appropriate Growth Hormone stimulation test.
Contraindications
Lyophilized Genotropin Powder should not be used if an individual shows any signs of neoplastic activity. Intracranial spade occupying lesions must be inert and antitumor treatment must be complete before Genotropin therapy begins. If the tumor begins to grow again, Genottropin treatment should be discontinued immediately. Also, Growth Hormone should not be administered as a means to promote growth in children who have fused epiphysis in the long bones.
Human Growth hormone treatments should not be administered to provide therapy for individuals who have an acutely critical illness stemming from abdominal or open-heart surgery, multiple physical accidental trauma, or to those who have suffered acute respiratory failure.
A pair of clinical research trials involving 522 total adult participants that showed no signs of Growth Hormone Deficiency who suffered from these conditions were conducted. The results of these two studies revealed that there was a significantly higher incidence of mortality (41.9% vs. 19.3%) affecting those who were treated with somatropin (5.3 to 8 mg/day dosage) in comparison to those that merely received placebo (read Warnings for more information).
Warnings
The 13.8 mg and 5.8 mg preparations of Lyophilized Genotropin Powder contain the preservative m-Cresol. Those who have a sensitivity or allergy to this preservative should not use these products. The Genotropin Miniquick and Genotropin 1.5 mg preparations do not have m-Cresol or any preservative (Read the How Supplied section for more information).
Read the Contraindications section for more information about increased mortality risk in those who suffer from critical illnesses in ICU because of  complications due to abdominal or open-heart surgery, multiple trauma due to an accident, or those who are suffering from acute respiratory failure. The proper research has not been established regarding the safety of continuing to undergo Growth Hormone Replacement Therapy for those who would be eligible for the treatment but also concurrently begin to suffer from the above ailments.
complications due to abdominal or open-heart surgery, multiple trauma due to an accident, or those who are suffering from acute respiratory failure. The proper research has not been established regarding the safety of continuing to undergo Growth Hormone Replacement Therapy for those who would be eligible for the treatment but also concurrently begin to suffer from the above ailments.
For this reason, the possible benefits of Growth Hormone Replacement Therapy in those patients who are suffering from critical, acute injuries must be weighed against the possible risks of hormone treatment. The pros and cons of hormone replacement therapy must be properly evaluated.
Precautions: Is it time to think about stopping hormone replacement therapy?
General
Therapy with Lyophilized Genotropin Powder should be guided by HRT doctors who have experience in the management and diagnosis of patients with Growth Hormone Disorder, as should be the case with the usage of any Growth Hormone preparation.
Caregivers and patients that will inject Genotropin in situations that are medically unsupervised are sternly advised to attain proper instruction and training about how to properly use Genotropin. This training should be given by a specialized doctor or other properly trained health professional.
Patients who suffer from Growth Hormone Disorder in addition to an intracranial lesion need to visit their doctor with regularity to monitor the development or recurrence of the underlying process of their disease. A survey of reports regarding the use of somatotropin replacement therapy shows no correlation between somatropin replacement therapy and tumors of the central nervous system. Later in life, there has been insufficient research to discover is there is any sort of causal relationship between the occurrence of Central Nervous System tumors and Somatropin Treatment
It is also vitally important that patients be carefully monitored for the formation of malignant lesions on the skin.
Caution is advised if Human Growth Hormone Therapy is administered to those that are already suffering from diabetes mellitus, because the level of insulin intake may need to be changed as a result of the hormone therapy. Those undergoing Growth Hormone Replacement Therapy should be monitored for the development of a glucose intolerance because Growth Hormone can sometimes give rise to a state where one is more resistant to insulin.
Those patients that have glucose intolerance or diabetes must be closely monitored while taking Genotropin. If major complications begin to arise it may be time to think about stopping hormone replacement therapy.
With those that have been diagnosed with hypopituitarism (a disease that is the result of multiple deficiencies of the hormonal system), the normal regimen of Hormone Replacement Therapy should be closely monitored when Genotropin treatment commences.
Hypothyroidism can develop as one is treated with Genotropin, and as a result, inadequate medical response to hypothyroidism can cause Genotropin to become less effective or ineffective. For this reason, it is pertinent that patients get tested for proper thyroid function periodically, and if the thyroid becomes less active, thyroid hormone should also be administered in tandem with Genotropin.
Pediatric patients who have disorders of the endocrine system such as Growth Hormone Disorder have been shown to have a greater risk of developing slipped capital femoral epiphyses. Any young patient that develops a limp or begins to complain of knee or hip pain as they undergo Growth Hormone Therapy needs to undergo evaluation.
Intracranial hypertension (IH) with nausea, vomiting, headache, visual disruption, and/or papilledema has been shown to be a side effect in a small minority of patients that take Growth Hormone therapies. These issues occur usually within the first two months after one begins GHT. In each reported case, and signs of Intracranial Hypertension are alleviated after hormone therapy is terminated or the dosage is adjusted to a more minute amount. Examination of the ocular fundus is recommended as a patient begins therapy, and it is also advised that they are examined for Intracranial Hypertension periodically as he or she continues Therapy.
Before undergoing adult treatment for Growth Hormone Deficiency, a patient who has undergone puberty and received GHRT in their childhood needs to be reevaluated in a manner that is recommended in the section Indications and Usage. If it is advisable to continue treatment, Genotropin therapy should continue at a lower level of dosage that is more appropriate for adults who have Growth Hormone Disorder.
Drug Interactions
Undergoing treatment with glucocorticoids concomitantly with Growth Hormone can cancel out the growth promotional effect of Hormone Therapy. Pediatric Growth Hormone deficiency patients that have a coexisting deficiency of ACTH need to have their dose adjusted carefully so that the glucocorticoid does not inhibit the effect of the Growth Hormone Replacement Therapy.
For more information, see Precautions. Though there is limited published research on the subject, it has been indicated that in humans, GHT increases antipyrine evacuation mediated by CP450 (cytochrome P450). The available data seems to suggest that Growth Hormone administration can alter the evacuation of the compounds that become metabolized through the liver enzyme CP450.
These compounds include sexual steroids, cyclosporine, anticonvulsants, and corticosteroids. It is advised that a patient undergo careful monitoring when Genotropin is injected in addition to other drugs that are metabolized by the liver enzyme CP450.
Mutagenesis, Carcinogenesis, and fertility Issues with Human Growth Hormone replacement therapy
Studies of carcinogenicity have yet to be conducted in regard to bio-identical Human Growth Hormone. A number of tests have shown no evidence of the mutagenicity of Human Growth Hormone. The tests conducted include the Ames Test in which gene mutations are introduced to bacteria, mutation of genes in mammalian cellular cultures grown in vitro (L5178Y cells), and damage to the chromosomes of intact animals (rat marrow cells). To learn more about issues regarding fertility, go to the section entitled Pregnancy.
Pregnancy category
Fertility studies enacted in regard to Genotropin at dosage levels of 0.3, 1, and 3.3 milligrams per kilogram per day delivered subcutaneously to rats and dosage levels of 0.08, 0.3, and 1.3 milligrams per kilogram per day delivered intramuscularly to rabbits resulted in a reduction in the body weight of the mother but the reduction was not teratogenic. The highest administered doses were about 24x and 19x the normal human levels for therapy, respectively, based upon the surface area of the body.
reduction was not teratogenic. The highest administered doses were about 24x and 19x the normal human levels for therapy, respectively, based upon the surface area of the body.
Rats received subcutaneous doses of Genotropin during gametogenesis and through the initial seven days of pregnancy at a rate of 3.3 mg/kg/day. This level is about 24x higher than the normal human dosage. This dosage produced extended cycles of estrous in females (known as anestrus), and fewer, less motile sperm in male rats.
When Genotropin was administered to female, pregnant rats during days one through seven of gestation at 3.3 mg/kg/day, a modest increase of fetal mortality occurred. When administered 1 mg/kg/day (which is around 7x the normal human dose) rats displayed estrus cycles which were only slightly extended. At a dosage of 0.3 mg/kg/day, there were no noted effects.
In postnatal and perinatal studies of rats, doses of Genotropin at 0.3, 1, and 3.3 mg/kg/day resulted in the promotion of growth of the dams but not of the fetuses, themselves. Young rats exposed to 3.3 mg/kg/day were discovered to have an increase in weight gain during the suckling period, but these effects were no longer apparent at ten weeks old.
There were no negative effects observed during morphogenesis, gestation, lactation, parturition, postnatal continued development, or the fertility of the offspring as a result of Genotropin.
It is important to note, however, that there have been no adequate, well-conducted studies of pregnant women in regard to Genotropin. Studies regarding animal reproduction do not always correctly predict the equivalent human response to the same stimulus, therefore, Genotropin should only be used during pregnancy if it is clearly and absolutely needed.
Mothers who are nursing
No studies have been conducted with regard to Genotropin and nursing mothers. At this point, it is not clear whether the drug is present in human milk
. Since it is a fact that a number of drugs are released in human milk, caution must be exercised with the administration of Genotropin to a nursing female.
Bio-identical hormone replacement therapy side effects
As with all drugs that are protein-derived, a small minority of patients can begin to manufacture antibodies to the administered protein. Growth Hormone antibody with a lower binding than 2 mg/L has not been shown to be associated with the attenuation of growth. In those cases where the capacity for binding is greater than 2 mg/L, antibodies sometimes interfere with the desired results for growth.
In a survey of 419 pediatric GHT patients who were administered Lyophilized Genotropin powder, 244 were previously treated with Genotropin, and 175 were undergoing GHT for the first time. Baseline presence of Anti-Growth Hormone antibodies had developed in 6 patients who were previously treated with Genotropin. 3 of those 6 once again tested negative for anti-growth Hormone antibodies during six to twelve months of Genotropin treatment.
Out of the rest of the group (413 patients), 8 (1.9% of the population) acquired detectable levels anti-Growth Hormone antibodies during Genotropin treatment. None of these antibodies reached a binding capacity of greater than 2mg/L. As a result, the patients who tested positive for Human Growth Hormone antibodies experienced no hindrance to Genotropin's intended growth response.
Genotropin preparations do contain trace amounts of periplasmic E. coli peptides (abbreviated PECP). Antibodies resistant to PECP develop in a small minority of those treated with Genotropin, but these antibodies do not create any issues of significance.
Clinical Genotropin studies with pediatric Growth Hormone Deficient patients uncommonly reported the following side effects: burning and pain around the  administration site, nodules, inflammation, fibrosis, rash, bleeding, or skin pigmentation. Other side effects include headache, hypothyroidism, lipoatrophy, hematuria, and mild hyperglycemia.
administration site, nodules, inflammation, fibrosis, rash, bleeding, or skin pigmentation. Other side effects include headache, hypothyroidism, lipoatrophy, hematuria, and mild hyperglycemia.
There have been a limited number of reports of leukemia among young patients who have undergone GHT including pituitary-derived Growth hormone and Bio-Synthetic somatropin. The correlation, if any, between GHT and leukemia is unclear at this time.
In a survey of clinical Genotropin trials in 1,145 adults with Growth Hormone Deficiency, the most reported side-effect was mild or moderate fluid retention resulting in hypoesthesia, paresthesia, myalgia, peripheral edema, stiffness and pain in the extremities, peripheral swelling, and arthralgia. The previous side effects were noted early in Genotropin Therapy and they often subsided with time or disappeared with a reduction in dosage.
Table 2 shows side effects reported by five per cent or more of adult patients with Growth Hormone Deficiency in a variety of clinical trial settings and after a number of different Genotropin treatment durations. Also, corresponding rates of side effect incidence are listed for those who underwent placebo treatment during the six-month portion of double-blind clinical trials.
Table 2
Side effects reported by greater than or equal to 5 per cent of a total of 1,145 Growth Hormone Deficient adult patients who participated in clinical administration of placebo and Genotropin, arranged by treatment duration, double-blind, and open-label phase. Percentages are rounded to the nearest 0.5%
Genotropin
Side-effects
Placebo Patients
0-6 months
n = 572
# Genotropin Patients
0-6 months
number of participants =573
Percentage patients six-twelvemonths
n = 63
Percentage of patients twelve-eighteen months
n = 63
Percentage of patients eighteen-twenty four months
n = 60
Percentage Patients Reporting SideEffect
Peripheral Swelling 5.0 / 17.5* / 5.5 / 0 / 1.5
Infection of the upper respiratory tract 14.5 / 15.5 / 13.0 / 15.0 / 13.5
Arthralgia 4.0 / 17.0* / 7.0 / 6.5 /3.5
Extremity stiffness 6.0 / 15.0* / 6.5 / 1.5 / 3.5
Tingling sensation 1.0 / 9.5* / 2.0 / 3.0 / 0
Peripheral edema 2.5 / 11.0* / 3.0 / 0 / 0
Extremity Stiffness 1.5 / 8.0* / 2.5 / 1.5 / 0
Headache 8.0 / 10.0 / 6.0 / 00
Pain reported in back 4.5 / 3.0 / 3.5 / 5.0 / 5.0
Muscle pain 1.5 / 5.0 * / 2.0 / 5.0 / 7.0
Fatigue 4.0 / 6.0 / 4.5 / 6.5 /1.5
* Significant increase in comparison to those taking placebo. P n =
# of patients that received treatment in the specific period.
% = Patient percentage who experienced the side effect in the specific period.
In an expanded study that occurred post-trial, diabetes was reported in twelve out of a total 3,031 patients as they were treated with Genotropin, only 0.4$ of the population. Each of the twelve patients showed risk factors for diabetes such as significant obesity or high levels of glycated hemoglobin, before beginning Genotropin Treatment.
Out of that same pool of 3,031 Genotropin-receiving patients, only 2% (61) developed carpal tunnel symptoms, and this number dropped by 52 after treatment interruption or dosage adjustment, and the other nine found their symptoms alleviated by surgery. Other side effects that were reported in the study included  hypoesthesia and general edema.
hypoesthesia and general edema.
Overdose
Little information is available regarding chronic or acute overdosage of Lyophilized Genotropin Powder. Growth Hormone administered intravenously has shown evidence that it results in a severe decrease in the plasma of the blood.
For this reason, hyperglycemia sometimes occurs as a result. It is hypothesized that this same issue could occur in the rare instance where a very high dose of Genotropin is administered subcutaneously. Overdosage over the long term could result in hormone imbalance symptoms and signs of acromegaly that are consistent with the nature of overproduction of Human Growth Hormone. Make sure to engage in safe hormone replacement therapy.
Administration and dosage
The appropriate dosage of lyophilized Genotropin Powder is dependent upon the individual needs of the patient. The dosage for the week ideally is divided into six or seven SC injections. The injection of Genotropin can be administered to the abdomen, buttocks, or thigh. The area where the subcutaneous injection occurs should change each day so that lipoatrophy is averted.
For Pediatric Growth Hormone Deficiency patients, a dosage of 0.16 to 0.24 milligrams per kilogram of body weight per week is generally the recommended dosage.
For Adult Growth Hormone Deficiency Patients, the initial recommended dose when one begins therapy is generally not greater than 0.04 mg/kg/week. Every four to eight weeks, the dosage can be increased as needed for the patient up to a max of 0.08 mg/kg/week dependent upon the patient's tolerance for the treatment.
Response to the medicine developed side effects, and levels of age-adjusted Insulin-Like Growth Factor-1 in serum can all be used to provide a guide leading to a proper titration of dosage. This regimen tends to lead to larger weight-adjusted treatment for women than men, and smaller weight-adjusted treatment for obese and older patients.
Do not inject Genotropin intravenously.
Genotropin is provided in a two-chambered cartridge, with the lyophilized powder located in the front compartment and a dilutant located in the rear compartment. A reconstitution device mixes the dilutant and the powder.
Each device has its own directions to follow for proper reconstitution. It is vital that the medicine is not shaken, because shaking can lead to denaturation of the primary ingredient.
Before injection, all parenteral medicinal products need to be visually inspected for discoloration and particulate matter, if the container and solution permit. If the solution has become cloudy, it is vital that the contents are not injected.
Caregivers and patients that will administer the Genotropin in situations in situations where they are not medically supervised are urged to visit one of many bioidentical hormone physicians other appropriately qualified health professionals in order to acquire the appropriate instruction and training regarding the proper administration of Genotropin.
Storage and Stability
Except in the proceeding situation, store Lyophilized Genotropin Powder in refrigeration at 2-8 degrees Celsius (36-46 degrees Fahrenheit). Refrain from freezing. Store in a light-protected area.
 The 1.5 mg Genotropin Cartridge contains a dilutent without a preservative. After the drug is reconstituted, a cartridge can be stored safely for up to a day. Use it only once and throw away any solution that remains.
The 1.5 mg Genotropin Cartridge contains a dilutent without a preservative. After the drug is reconstituted, a cartridge can be stored safely for up to a day. Use it only once and throw away any solution that remains.
The 13.8 and 5.8 mg Genotropin cartridges contain a diluent that is laced with a preservative. For this reason, these cartridges may be stored in refrigeration for as long as three weeks.
The Genotropin Miniquick Growth Hormome Delivery Device needs to be refrigerated before it is dispensed, but it can be placed in storage at a temperature of 25 degrees Celsius (77 degrees Fahrenheit) or less for a period of as long as 3 months after it is dispensed. The dilution does not contain preservatives. After the Genotropin Miniquick has been reconstituted, it may be placed in storage under refrigeration for up to one day before use. After it is used one time it should be discarded.
How Supplied
Lyophilized Genotropin Powder is available for purchase in the following configurations:
1.5-milligram two-chambered cartridge (without preservative)
- mg/mL concentration (about 4IU/mL)
Preconfigured in a Genotropin Intramix GH Reconstitution Device which is packaged with a single pressure release needle
Five Cartridge Package National Drug Code 0013-2606-94
5.8-milligram two-chambered container (added preservative)
5 mg/mL concentration (about 15IU/mL)
Intended for usage with the Genotropin Mixer GH Reconstitution device and/or the Genotropin Pen 5 GH Delivery Device
Five Cartridge package National Drug Code 0013-2626-94
One Cartridge package National Drug Code 0013-2626-81
Preconfigured in a Genotropin Intramix GH Device for Reconstitution and packaged with one needle which functions through pressure release.
Package of five National Drug Code0013-2616-94
Package of one National Drug Code0013-2616-81
13.8 milligram two-chambered container (added preservative)
12 mg./mL concentration (about 36IU/mL)
Intended for usage with the Genotropin Mixer GH Reconstitution device and/or the Genotropin Pen 12 GH Delivery Device
Box of Five National Drug Code0013-2646-94
Box of One National Drug Code0013-2646-81
Genotropin Miniquick GH Delivery Device contains a two-compartment cartridge containing an a two-chamber cartridge of GENOTROPIN (no preservative)
After it is reconstituted, every Genotropin Miniquick released a 0.25 mL fixed dose without regard to strength. It is available in the proceeding strengths in packages of seven.
0.2 milligram National Drug Code0013-2649-02
0.4 milligram National Drug Code0013-2650-02
0.6 milligram National Drug Code0013-2651-02
0.8 milligram National Drug Code0013-2652-02
1.0 milligram National Drug Code0013-2653-02
1.2 milligram National Drug Code0013-2654-02
1.4 milligram National Drug Code0013-2655-02
1.6 milligram National Drug Code0013-2656-02
1.8 milligram National Drug Code0013-2657-02
2.0 milligram National Drug Code0013-2658-02
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Injectable HGH Prescriptions: Blood Testing Centers in Philadelphia, Pennsylvania [Last Updated On: February 26th, 2024] [Originally Added On: March 3rd, 2019]
- Nutropin Hgh: Hgh Hormone Product [Last Updated On: December 21st, 2024] [Originally Added On: March 10th, 2020]
- Hgh Replacement [Last Updated On: December 21st, 2024] [Originally Added On: March 14th, 2020]
- Hgh Therapy [Last Updated On: December 22nd, 2024] [Originally Added On: March 15th, 2020]
- Anti-ageing Clinics Distribute Human Growth Hormone [Last Updated On: December 25th, 2024] [Originally Added On: March 16th, 2020]
- Hgh In Forefront To Remain Young [Last Updated On: November 25th, 2024] [Originally Added On: March 18th, 2020]
- Benefits Of Hgh: Thierry Hertoghe [Last Updated On: December 23rd, 2024] [Originally Added On: April 23rd, 2020]
- Getting Started With An Hgh Program [Last Updated On: December 24th, 2024] [Originally Added On: April 25th, 2020]
- Hgh Releasers [Last Updated On: December 25th, 2024] [Originally Added On: April 26th, 2020]
- What dietary considerations should I make while on HRT, hormone replacement therapy? [Last Updated On: December 20th, 2024] [Originally Added On: May 1st, 2020]
- Hgh Dosage [Last Updated On: March 3rd, 2025] [Originally Added On: May 5th, 2020]
- Hgh Deficiency [Last Updated On: December 23rd, 2024] [Originally Added On: May 6th, 2020]
- Hgh Products Genotropin Humantrope Nutropin Saizen Serostim Omnitrope [Last Updated On: November 25th, 2024] [Originally Added On: May 8th, 2020]
- What Is Injectable Hgh Human Growth Hormone? [Last Updated On: December 22nd, 2024] [Originally Added On: May 9th, 2020]
- Introduction To Human Growth Hormone HGH [Last Updated On: December 24th, 2024] [Originally Added On: May 10th, 2020]
- Omnitrope Injections for the Treatment of HGH Deficiency [Last Updated On: July 7th, 2024] [Originally Added On: May 6th, 2021]
- Sleep To Amplify Human Growth Hormone Levels [Last Updated On: July 6th, 2024] [Originally Added On: May 27th, 2021]
- Buying Hgh Hormone Human Growth Hormone [Last Updated On: July 19th, 2024] [Originally Added On: February 22nd, 2022]
- Hgh For Menopause [Last Updated On: September 27th, 2024] [Originally Added On: February 23rd, 2022]
- Your HGH Levels [Last Updated On: September 4th, 2024] [Originally Added On: February 24th, 2022]
- Related Glossary of Terms [Last Updated On: October 14th, 2024] [Originally Added On: February 25th, 2022]
- Hgh Facts - Information About Growth Hormone [Last Updated On: July 20th, 2024] [Originally Added On: February 26th, 2022]
- Hgh Sports And Baseball [Last Updated On: July 21st, 2024] [Originally Added On: February 27th, 2022]
- Us Olympic Committee And Talk Of Hgh [Last Updated On: September 29th, 2024] [Originally Added On: February 28th, 2022]
- Braves' Schafer Talks About His Suspension Over Hgh [Last Updated On: July 22nd, 2024] [Originally Added On: March 1st, 2022]
- Hgh Scams: Different Hgh Scams And Things To Watch For Online [Last Updated On: July 26th, 2024] [Originally Added On: March 2nd, 2022]
- Nordiflex HGH Injection Device Information [Last Updated On: September 30th, 2024] [Originally Added On: March 3rd, 2022]
- Saizen Hgh Detailed Description [Last Updated On: October 17th, 2024] [Originally Added On: March 6th, 2022]
- Genotropin HGH: Human Growth Hormone Product [Last Updated On: July 23rd, 2024] [Originally Added On: March 6th, 2022]
- Omnitrope Hgh: Human Growth Hormone Product Called Omnitrope. [Last Updated On: October 5th, 2024] [Originally Added On: March 7th, 2022]
- Nutropin Hgh Detailed Description [Last Updated On: October 3rd, 2024] [Originally Added On: March 8th, 2022]
- Saizen Hgh: Injectable Hgh Product [Last Updated On: July 24th, 2024] [Originally Added On: March 9th, 2022]
- Hgh Product Serostim [Last Updated On: July 25th, 2024] [Originally Added On: March 11th, 2022]
- HGH Scams (HGH Supplements) [Last Updated On: November 12th, 2024] [Originally Added On: March 20th, 2022]
- Side Effects Of HGH [Last Updated On: August 2nd, 2024] [Originally Added On: March 21st, 2022]
- Testimonial: Walter’s HGH Story [Last Updated On: August 28th, 2024] [Originally Added On: June 4th, 2022]
- HGH Is the “God Particle” [Last Updated On: August 17th, 2024] [Originally Added On: July 26th, 2022]
- Low testosterone in men might increase the risk of severe COVID [Last Updated On: September 20th, 2024] [Originally Added On: September 6th, 2022]
- Can I Reverse the Aging Process with HGH? [Last Updated On: August 19th, 2024] [Originally Added On: September 21st, 2022]
- A new study confirms: Testosterone Replacement Therapy is safe! [Last Updated On: September 3rd, 2024] [Originally Added On: October 2nd, 2022]
- HGH Therapy – How Can I Know Who Is Legitimate? [Last Updated On: June 29th, 2024] [Originally Added On: January 10th, 2023]
- The Evolution of Testosterone Replacement Therapy for men with Prostate Cancer [Last Updated On: June 24th, 2024] [Originally Added On: January 12th, 2023]
- Nine Hormones that Can Impact Your Weight and How to Promote Hormone Balance [Last Updated On: June 28th, 2024] [Originally Added On: January 26th, 2023]
- Get Tested for Hormone Levels, even if You Have No Symptoms [Last Updated On: August 29th, 2024] [Originally Added On: March 17th, 2023]
- Can Head Injuries Cause HGH Deficiency? [Last Updated On: August 26th, 2024] [Originally Added On: May 17th, 2023]
- Mountain Biker Thought his Life Was Over [Last Updated On: August 15th, 2024] [Originally Added On: May 25th, 2023]
- Do HGH Booster Supplements Work Faster Than Prescriptions? [Last Updated On: August 31st, 2024] [Originally Added On: June 3rd, 2023]
- HGH and It’s Effects on Other Hormones [Last Updated On: October 26th, 2024] [Originally Added On: June 10th, 2023]
- Maintaining a Proper Medication Schedule [Last Updated On: November 24th, 2024] [Originally Added On: August 23rd, 2023]
- What is the History of the Discovery of HGH? [Last Updated On: October 21st, 2024] [Originally Added On: September 17th, 2023]
- Don’t Let Fear Stop You From Fixing Your Hormones [Last Updated On: October 22nd, 2024] [Originally Added On: September 22nd, 2023]
- Understanding HGH Dosage [Last Updated On: February 26th, 2025] [Originally Added On: February 26th, 2025]