Introduction
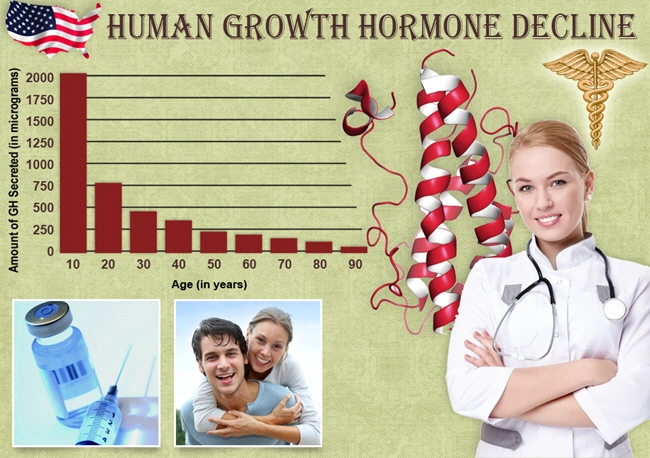
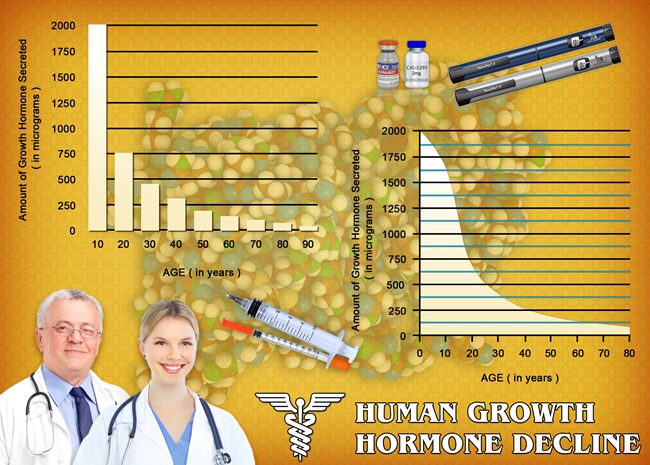
Non-Alcoholic Fatty Liver Disease (NAFLD) represents a significant health concern among American males, characterized by excessive fat accumulation in the liver not caused by alcohol consumption. This condition can progress to more severe forms such as non-alcoholic steatohepatitis (NASH), cirrhosis, and even hepatocellular carcinoma. In the search for effective treatments, the potential of Ipamorelin, a growth hormone secretagogue, has been explored. This article delves into the findings of a three-year clinical trial investigating the effects of Ipamorelin on liver function in American males diagnosed with NAFLD.
Study Design and Methodology
The clinical trial was conducted over three years, involving a cohort of 200 American males aged 30 to 65, all diagnosed with NAFLD. Participants were randomly assigned to either the treatment group, receiving daily doses of Ipamorelin, or the control group, receiving a placebo. Liver function was assessed through regular monitoring of liver enzyme levels, imaging studies, and liver biopsy where necessary. The primary aim was to evaluate changes in liver fat content and overall liver health metrics.
Results: Liver Enzyme Levels
A significant finding of the study was the reduction in liver enzyme levels among participants treated with Ipamorelin. Specifically, levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), which are indicative of liver damage, showed a statistically significant decrease in the treatment group compared to the placebo group. This suggests that Ipamorelin may have a protective effect on the liver, potentially reducing inflammation and damage associated with NAFLD.
Results: Liver Fat Content
Imaging studies, including MRI and ultrasound, were used to assess changes in liver fat content. The treatment group exhibited a notable reduction in hepatic steatosis, with an average decrease of 15% in liver fat content over the three-year period. This improvement was not observed in the control group, highlighting the potential of Ipamorelin in reducing fat accumulation in the liver.
Results: Fibrosis and Inflammation
Liver biopsies performed at the end of the trial provided insights into the effects of Ipamorelin on liver fibrosis and inflammation. The treatment group showed less progression of fibrosis and reduced signs of inflammation compared to the control group. These findings suggest that Ipamorelin may help mitigate the progression of NAFLD to more severe conditions such as NASH and cirrhosis.
Safety and Tolerability
Throughout the trial, Ipamorelin was well-tolerated by participants, with no serious adverse events reported. Common side effects included mild gastrointestinal discomfort and headaches, which resolved without intervention. These results indicate that Ipamorelin is a safe option for long-term use in the management of NAFLD.
Implications for Clinical Practice
The findings of this clinical trial suggest that Ipamorelin could be a valuable addition to the treatment regimen for American males with NAFLD. By reducing liver enzyme levels, decreasing liver fat content, and mitigating fibrosis and inflammation, Ipamorelin offers a multifaceted approach to managing this prevalent condition. Further studies are warranted to confirm these findings and explore the optimal dosing and duration of treatment.
Conclusion
The three-year clinical trial investigating the effects of Ipamorelin on liver function in American males with NAFLD has provided promising results. The observed improvements in liver enzyme levels, liver fat content, and signs of fibrosis and inflammation highlight the potential of Ipamorelin as a therapeutic agent. As NAFLD continues to pose a significant health challenge, the integration of Ipamorelin into treatment strategies could offer new hope for affected individuals. Continued research will be crucial in fully understanding the benefits and limitations of this promising treatment.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Empowering Your Body's Potential: An Insight into Ipamorelin [Last Updated On: February 25th, 2025] [Originally Added On: February 25th, 2025]
- Ipamorelin: Enhancing HGH for Vitality and Health in American Males [Last Updated On: February 26th, 2025] [Originally Added On: February 26th, 2025]
- Unleashing a Health Catalyst: Ipamorelin's Groundbreaking Impact on Wellness and Fitness [Last Updated On: February 26th, 2025] [Originally Added On: February 26th, 2025]
- Mastering your Intrinsic Capability: A Deep Dive into Ipamorelin [Last Updated On: February 27th, 2025] [Originally Added On: February 27th, 2025]
- Deciphering the Ipamorelin Paradigm: A Scientific Examination on its Propensity to Galvanize Endogenous HGH [Last Updated On: February 28th, 2025] [Originally Added On: February 28th, 2025]
- Unveiling the Potential of Body Transformation: The Rise and Evolution of Ipamorelin [Last Updated On: February 28th, 2025] [Originally Added On: February 28th, 2025]
- Deciphering Peptides: An In-depth Comparison of Ipamorelin and Sermorelin [Last Updated On: March 1st, 2025] [Originally Added On: March 1st, 2025]
- Introduction To Peptide Hormone Therapies [Last Updated On: March 2nd, 2025] [Originally Added On: March 2nd, 2025]
- Exploring Ipamorelin: A Selective Growth Hormone Secretagogue for Anti-Aging Applications [Last Updated On: March 3rd, 2025] [Originally Added On: March 3rd, 2025]
- Optimal Ipamorelin Dosing and Administration for American Men [Last Updated On: March 4th, 2025] [Originally Added On: March 4th, 2025]
- Exploring Ipamorelin: Enhancing Fat Loss and Muscle Growth Safely [Last Updated On: March 5th, 2025] [Originally Added On: March 5th, 2025]
- Exploring Ipamorelin: Benefits and Considerations for Muscle Growth in American Males [Last Updated On: March 6th, 2025] [Originally Added On: March 6th, 2025]
- Ipamorelin: A Breakthrough Recovery Solution for American Males in Medical Treatments [Last Updated On: March 7th, 2025] [Originally Added On: March 7th, 2025]
- Ipamorelin: Enhancing Athletic Performance Safely for American Male Athletes [Last Updated On: March 8th, 2025] [Originally Added On: March 8th, 2025]
- Ipamorelin: Natural HGH Enhancement for Men's Vitality Without Synthetic Side Effects [Last Updated On: March 9th, 2025] [Originally Added On: March 9th, 2025]
- Unveiling Ipamorelin: A Breakthrough in Muscle Enhancement and Fat Reduction for American Males [Last Updated On: March 10th, 2025] [Originally Added On: March 10th, 2025]
- Unlocking the Fountain of Youth: Exploring the Anti-Aging Potential of Ipamorelin [Last Updated On: March 12th, 2025] [Originally Added On: March 12th, 2025]
- Exploring the Potential of Ipamorelin: A Breakthrough in Peptide Therapy for American Males [Last Updated On: March 12th, 2025] [Originally Added On: March 12th, 2025]
- Revolutionizing Fitness: Personalized Peptide Therapy with Ipamorelin for American Males [Last Updated On: March 13th, 2025] [Originally Added On: March 13th, 2025]
- Unlocking the Potential of Ipamorelin: A Comprehensive Guide for American Males [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Ipamorelin: Enhancing Post-Workout Recovery for American Males [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Ipamorelin: Enhancing Men's Health through Selective GH Stimulation and Hormonal Balance [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Ipamorelin Therapy: Cost-Effectiveness and Benefits for American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Ipamorelin: Enhancing Sleep Quality and Recovery in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Ipamorelin and Intermittent Fasting: Synergistic Metabolic Optimization for American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Ipamorelin: Enhancing Muscle Growth, Sleep, and Vitality in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Ipamorelin: Enhancing Fitness in American Men Through Selective Growth Hormone Stimulation [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Ipamorelin: A Key to Anti-Aging for American Males [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Ipamorelin: Enhancing Regenerative Medicine for American Men's Health [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Ipamorelin: Enhancing Muscle Growth and Performance in American Male Athletes [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Ipamorelin: Enhancing Muscle, Bone, and Recovery in American Males - Case Studies [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Ipamorelin: A Promising Peptide for Combating Fatigue in American Males [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Ipamorelin Therapy: Enhancing Benefits with Optimal Nutrition for American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Ipamorelin: Enhancing Hormonal Balance in American Males - A Comprehensive Guide [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Ipamorelin: Enhancing Hormonal Health in American Males Through Lifestyle Hacks [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Ipamorelin: Enhancing Metabolism and Fat Burning in American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Ipamorelin: Enhancing Health and Wellness in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Ipamorelin: Enhancing Health in American Males Through Peptide Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Ipamorelin: A Promising Peptide for Cardiovascular Health in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Ipamorelin: Enhancing Post-Workout Recovery for American Male Athletes [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Ipamorelin: Enhancing Male Vitality and Health Through Selective Growth Hormone Stimulation [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Tracking Ipamorelin Progress: Goals, Body Composition, and Hormonal Levels for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Ipamorelin: Revolutionizing Hormone Optimization for American Males' Health and Vitality [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Ipamorelin: Enhancing Muscle, Bone, Sleep, and Fat Loss in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Ipamorelin: Enhancing Performance and Recovery in American Male Athletes [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Ipamorelin: Enhancing Health and Longevity in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Ipamorelin: Enhancing Anti-Aging with Holistic Wellness Strategies for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Ipamorelin: Enhancing Tissue Repair and Regeneration in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Stress, Cortisol, and Hormonal Balance: Ipamorelin's Role in Men's Health [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Ipamorelin: Enhancing Post-Injury Recovery in American Males Through Selective GH Stimulation [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Ipamorelin Therapy: Benefits, Side Effects, and Management for American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Ipamorelin: Enhancing Longevity and Vitality in American Men Through Selective GH Release [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Ipamorelin Pharmacokinetics: Absorption, Metabolism, and Clinical Use in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Ipamorelin: Enhancing Growth and Vitality in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Ipamorelin Boosts Muscle Growth and Recovery in Resistance Training for American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Ipamorelin: Enhancing HGH for Muscle, Bone, and Metabolic Health in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Ipamorelin Therapy: Personalization for Enhanced Performance and Health in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Ipamorelin: Boosting Vitality and Performance in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Ipamorelin: Enhancing Bone Density in American Men Through GH Stimulation [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Ipamorelin: Enhancing Tissue Regeneration in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Ipamorelin: Enhancing GH Levels and Health in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Ipamorelin: Enhancing Muscle Growth and Performance in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Ipamorelin: Enhancing Health and Vitality in American Males Through GH Stimulation [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Ipamorelin: Enhancing Muscle, Bone, and Metabolic Health in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Ipamorelin: Enhancing Muscle Growth and Health in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Ipamorelin: Enhancing Hormonal Health and Circadian Rhythm in American Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Ipamorelin: Enhancing Fitness and Muscle Growth in American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Ipamorelin: A Safer, Effective GH Therapy Alternative for American Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Ipamorelin: A Selective Growth Hormone Secretagogue for Anti-Aging in American Males [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Ipamorelin: Enhancing Athletic Performance and Recovery in American Male Athletes [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Ipamorelin: Enhancing Muscle Growth and Performance in American Males [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Ipamorelin: Enhancing Cognitive Function and Mood in American Males [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Ipamorelin: Enhancing Vitality and Regeneration in American Males [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Ipamorelin: Enhancing Hormonal Health and Vitality in American Men [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Ipamorelin: Enhancing Muscle, Bone, and Metabolic Health in American Males [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Ipamorelin Therapy: Trends, Innovations, and Benefits for American Males [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Ipamorelin: Enhancing Endurance and Recovery in American Male Athletes [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Ipamorelin: A Targeted Approach to GH Stimulation for American Males' Health [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Ipamorelin: Enhancing Performance and Health in American Males - Usage and Best Practices [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Maximizing Ipamorelin Benefits: Diet, Exercise, Sleep, Stress, and Hydration for American Males [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]