Introduction
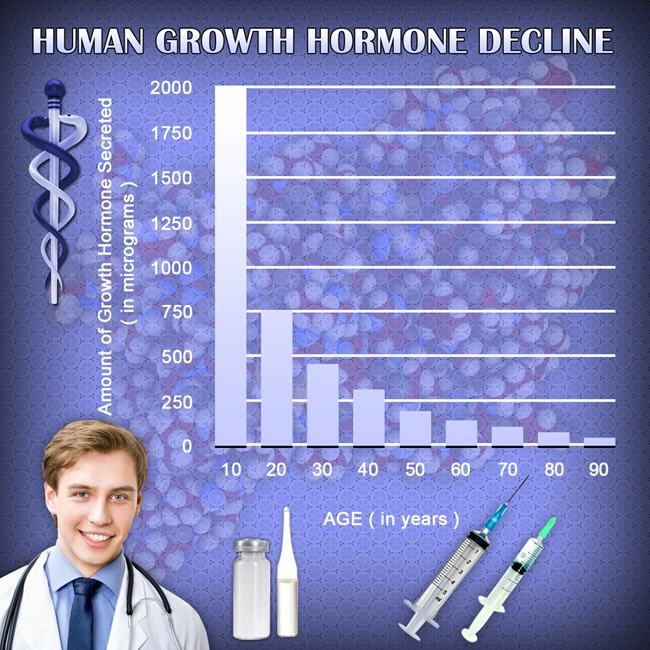
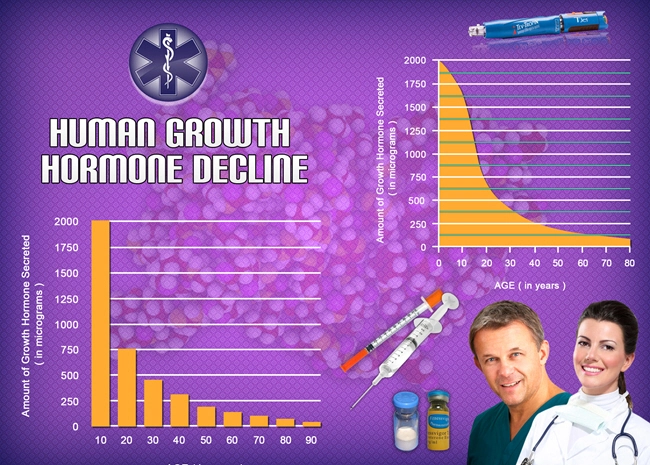
Humatrope, a synthetic form of human growth hormone (hGH), has been widely utilized in the treatment of growth hormone deficiency and other related conditions. As its application extends over long periods, understanding the safety and tolerability of Humatrope in American males becomes crucial. This article reviews the adverse events associated with long-term Humatrope administration over a decade, providing insights into its safety profile.
Overview of Humatrope and Its Uses
Humatrope, a recombinant human growth hormone, is primarily used to treat children and adults with growth hormone deficiency. It is also approved for other conditions, such as Turner syndrome and chronic kidney disease in pediatric patients. The long-term administration of Humatrope necessitates a thorough evaluation of its safety, especially in American males who may have different health profiles and lifestyle factors influencing drug response.
Methodology of the Review
This review encompasses data collected from clinical trials, observational studies, and post-marketing surveillance over a decade. The focus was on American males aged 18 and above, who received Humatrope for various indications. Adverse events were categorized and analyzed to assess the frequency, severity, and potential risk factors associated with long-term use.
Common Adverse Events
The most frequently reported adverse events in American males using Humatrope long-term include injection site reactions, such as pain, swelling, and erythema. These are generally mild and transient, resolving without the need for medical intervention. Other common side effects include headaches, joint and muscle pain, and fluid retention, which may manifest as swelling in the extremities.
Serious Adverse Events
While rare, serious adverse events have been reported. These include increased intracranial pressure, which can lead to symptoms such as severe headaches, nausea, and visual changes. In some cases, Humatrope has been associated with the development or progression of diabetes mellitus, particularly in individuals with risk factors for the disease. Additionally, there have been reports of neoplasms, although a direct causal link to Humatrope remains unestablished.
Long-Term Safety Concerns
Long-term administration of Humatrope raises concerns about potential impacts on cardiovascular health. Some studies suggest a possible association with increased risk of cardiovascular events, such as hypertension and myocardial infarction, though these findings are not consistent across all research. Monitoring of cardiovascular risk factors is recommended for patients on long-term Humatrope therapy.
Impact on Bone Health
Humatrope's effect on bone health is another area of interest. While growth hormone can promote bone growth, excessive or prolonged use may lead to changes in bone density and structure. Regular monitoring of bone health through imaging and biochemical markers is advised for patients on long-term therapy.
Psychological and Quality of Life Considerations
The psychological impact of long-term Humatrope use should not be overlooked. Some patients report improved quality of life due to enhanced physical capabilities and appearance, while others may experience psychological distress related to the chronic nature of their treatment and associated side effects. Comprehensive care that includes psychological support is essential for managing long-term Humatrope therapy.
Regulatory and Clinical Recommendations
Based on the reviewed data, regulatory bodies and clinicians should consider the following recommendations:
- Regular monitoring of patients for common and serious adverse events.
- Tailored risk assessment and management plans, particularly for individuals with pre-existing conditions.
- Education of patients on the potential risks and benefits of long-term Humatrope use.
- Continued research to further elucidate the long-term safety profile of Humatrope in diverse populations.
Conclusion
The long-term use of Humatrope in American males is generally well-tolerated, with most adverse events being mild and manageable. However, serious adverse events, though rare, necessitate vigilant monitoring and comprehensive patient management. As Humatrope continues to be a vital treatment option, ongoing research and surveillance are essential to ensure its safe and effective use in the long term.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Unveiling Strategies to Enhance Humatrope Therapy Compliance in Pediatric Care [Last Updated On: February 24th, 2025] [Originally Added On: February 24th, 2025]
- Humatrope's Role in Treating Growth Retardation in American Males with CKD [Last Updated On: February 25th, 2025] [Originally Added On: February 25th, 2025]
- Exploring the Role of Humatrope in Managing Short Bowel Syndrome: A Comprehensive Review [Last Updated On: March 7th, 2025] [Originally Added On: March 7th, 2025]
- Exploring the Impact of Humatrope on Quality of Life in Men with Growth Hormone Deficiency [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Unveiling the Impact of Humatrope on Lipid Profiles in Growth Hormone Deficient American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Immunomodulatory Effects of Humatrope in Men with Growth Hormone Deficiency [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Impact of Humatrope on Carcinoid Syndrome in Growth Hormone Deficient Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Impact of Humatrope Therapy on Vision in American Males with Growth Hormone Deficiency [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Therapeutic Potential of Humatrope in Treating Sheehan's Syndrome: A Clinical Perspective [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Humatrope Therapy Enhances Sleep Patterns in American Men with Growth Hormone Deficiency [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Humatrope Enhances Muscle Strength in Growth Hormone Deficient American Males: A Review [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Humatrope Therapy: Enhancing Cardiovascular Health in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Humatrope: Enhancing Wound Healing in American Males - Clinical Insights and Future Prospects [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Humatrope: Enhancing Growth and Life Quality in American Males with Noonan Syndrome [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Humatrope's Impact on Cognitive Function in American Males with Growth Hormone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Humatrope's Impact on Insulin Sensitivity in Men with Growth Hormone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Humatrope's Role in Managing HIV-Associated Wasting in American Males [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Humatrope: A Key Therapy for Growth Deficiencies in SGA Infants [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Humatrope: Safe, Effective Growth Hormone Therapy for American Males with Growth Disorders [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Humatrope: Boosting Energy and Vitality in American Males with Growth Hormone Deficiency [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Humatrope's Impact on Metabolic Syndrome in American Males with GHD: Efficacy and Safety [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Humatrope's Role in Managing Cachexia Among American Male Cancer Patients [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope: Anti-Aging Potential and Risks for American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope's Impact on Hair Growth in American Men with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope Therapy Enhances Dental Development in American Boys with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope Therapy's Impact on Vision in Growth Hormone Deficient American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope's Potential in Managing Hyperthyroidism: Insights for American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope Therapy's Impact on Adrenal Function in Growth Hormone Deficient American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Humatrope Therapy's Impact on Renal Function in American Males with GHD [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Humatrope Enhances Immune Function in American Men with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Humatrope's Role in Managing Cystic Fibrosis: Focus on American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Humatrope: A Beacon of Hope for American Males with SHOX Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope Therapy: A Promising Treatment for Chronic Fatigue Syndrome in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Impact on Mental Health in American Men with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope: Managing Pituitary Tumors and Growth Hormone Deficiency in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Impact on Gastrointestinal Function in American Men with GHD [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Impact on Fertility in American Males with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Impact on Parathyroid Function in American Males with GHD [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Impact on Thyroid Function in American Males with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope Therapy for Growth Hormone Deficiency Post-Cranial Irradiation in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope: Enhancing Growth and Health in American Males with GH Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope: Enhancing Bone Health in Males with Osteoporosis and Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope Therapy Enhances Skin Health in American Males with GHD [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Potential Benefits for American Males with Chronic Liver Disease [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Humatrope Therapy and Adrenal Insufficiency in American Males with GHD: A Comprehensive Analysis [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Humatrope's Role in Managing Congenital Adrenal Hyperplasia in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Humatrope: A Novel Treatment for Hypoparathyroidism in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Humatrope's Potential in Treating Rheumatoid Arthritis in American Males: An Overview [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Humatrope Enhances Pulmonary Function in Men with Growth Hormone Deficiency: A Review [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Humatrope: Enhancing Diabetes Management in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Humatrope's Role in Treating Anorexia Nervosa Among American Males: Benefits and Considerations [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Humatrope Therapy Enhances Hypothalamic Function in American Males with GHD [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Humatrope Therapy: A Promising Approach for Hyperparathyroidism in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Humatrope's Role in Managing Hypergonadism: Insights for American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Humatrope's Impact on Hearing in American Males with Growth Hormone Deficiency [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Humatrope: A Promising Adjunct Therapy for Hypothyroidism in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Humatrope's Role in Managing Addison's Disease: Benefits for American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Humatrope's Impact on Gonadal Function in American Males with Growth Hormone Deficiency [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Humatrope: Treating Growth Hormone Deficiency and Cushing's Syndrome in American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Humatrope's Role in Managing Hypogonadism: Insights for American Males [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Humatrope's Role in Treating GHD and Conn's Syndrome in American Males [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Humatrope Therapy: A Novel Approach to Managing Pheochromocytoma [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Humatrope's Therapeutic Impact on Carcinoid Syndrome in American Males with GHD [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Humatrope's Impact on Gigantism in American Males with Growth Hormone Deficiency [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Humatrope Therapy: Managing GHD and Acromegaly in American Males [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Humatrope's Role in Enhancing Neuroendocrine Tumor Treatment and Quality of Life [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Humatrope's Role in Treating Craniopharyngioma in American Males: A Comprehensive Guide [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Humatrope's Potential in Managing Multiple Endocrine Neoplasia Syndromes: A Comprehensive Overview [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Humatrope as Adjunct Therapy for Prolactinoma: Mechanisms, Clinical Use, and Future Research [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Humatrope Therapy: A New Hope for American Males with Diabetes Insipidus [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Humatrope's Role in Managing Sheehan's Syndrome: Insights for American Males [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Humatrope: A Vital Treatment for Growth Hormone Deficiency in American Males Post-Pituitary Apoplexy [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Humatrope's Impact on Central Precocious Puberty in American Males with GHD [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Humatrope Therapy's Impact on Empty Sella Syndrome in American Males with GHD [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Humatrope's Impact on Lymphocytic Hypophysitis in Men with Growth Hormone Deficiency [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Humatrope's Role in Treating SIADH: A Focus on American Males [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Humatrope Therapy: Enhancing Growth and Development in American Males with Down Syndrome [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Humatrope's Impact on Growth Hormone Deficiency in American Males with CHARGE Syndrome [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Humatrope's Impact on Prader-Willi Syndrome in American Males: Growth, Health, and Quality of Life [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Humatrope's Impact on Kallmann Syndrome and Growth Hormone Deficiency in American Males [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]