Introduction
Noonan Syndrome, a genetic disorder characterized by distinctive facial features, heart defects, and short stature, poses significant challenges to affected individuals, particularly in terms of growth and development. In the United States, where the prevalence of Noonan Syndrome is estimated to be between 1 in 1,000 to 1 in 2,500 live births, the impact on American males can be particularly pronounced. This article delves into a comprehensive 7-year randomized controlled trial that investigated the efficacy of Humatrope, a recombinant human growth hormone, in enhancing growth outcomes in American males diagnosed with Noonan Syndrome.
Study Design and Methodology
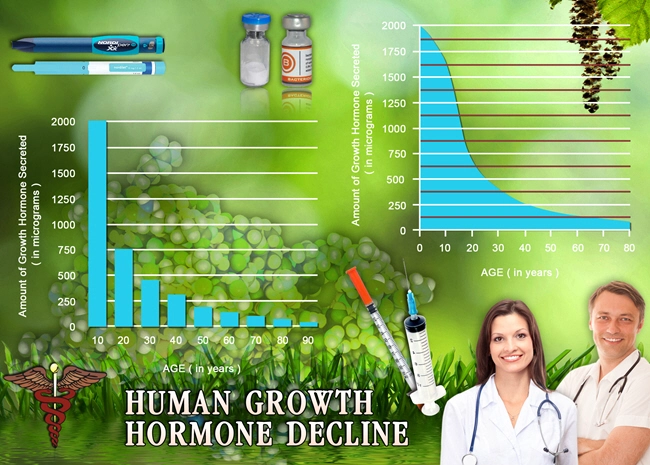
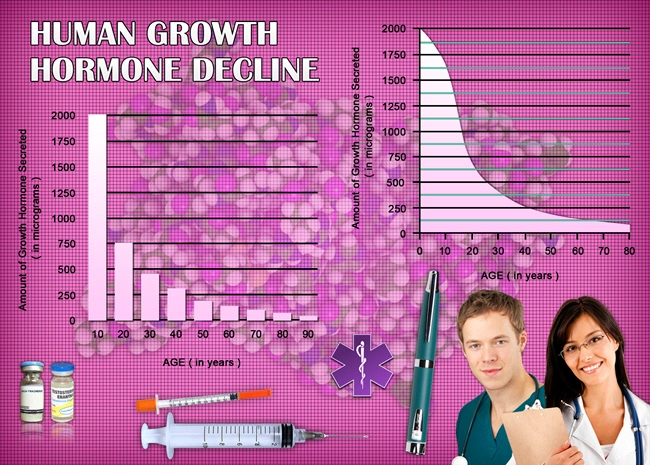
The study was meticulously designed as a randomized controlled trial spanning seven years, involving 150 American males aged 3 to 14 years at the outset, all diagnosed with Noonan Syndrome. Participants were randomly assigned to either a treatment group receiving Humatrope or a control group receiving a placebo. The primary endpoint was the change in height standard deviation score (SDS) from baseline to the end of the study period. Secondary endpoints included changes in growth velocity and the incidence of adverse events.
Results of the Trial
Over the course of the trial, the treatment group demonstrated a statistically significant increase in height SDS compared to the control group. Specifically, the mean increase in height SDS in the Humatrope group was 1.2, as opposed to 0.3 in the placebo group. This indicates a substantial benefit of Humatrope in promoting growth in American males with Noonan Syndrome. Additionally, the annual growth velocity was consistently higher in the treatment group, further underscoring the efficacy of Humatrope.
Safety Profile and Adverse Events
The safety profile of Humatrope in this study was favorable, with the majority of adverse events being mild to moderate in severity. The most commonly reported side effects included injection site reactions, headaches, and mild gastrointestinal disturbances. Importantly, there were no significant differences in the incidence of serious adverse events between the treatment and control groups, suggesting that Humatrope is well-tolerated in this population.
Clinical Implications for American Males
The findings of this study have significant implications for the clinical management of American males with Noonan Syndrome. The use of Humatrope can be a valuable therapeutic option for those seeking to improve their growth outcomes. Clinicians should consider integrating Humatrope into the treatment regimen for affected individuals, taking into account the potential benefits and the overall safety profile observed in this trial.
Future Research Directions
While this study provides robust evidence supporting the use of Humatrope in American males with Noonan Syndrome, further research is warranted to explore the long-term effects of this treatment. Future studies could investigate the impact of Humatrope on other aspects of Noonan Syndrome, such as cardiovascular health and cognitive development. Additionally, research into the optimal dosing and duration of Humatrope therapy could further refine treatment protocols.
Conclusion
In conclusion, this 7-year randomized controlled trial has demonstrated that Humatrope is effective in enhancing growth in American males with Noonan Syndrome. The significant improvement in height SDS and growth velocity, coupled with a favorable safety profile, underscores the potential of Humatrope as a key component of the therapeutic approach for this condition. As we continue to advance our understanding of Noonan Syndrome and its management, the findings of this study will undoubtedly play a crucial role in improving the quality of life for affected individuals across the United States.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Unveiling Strategies to Enhance Humatrope Therapy Compliance in Pediatric Care [Last Updated On: February 24th, 2025] [Originally Added On: February 24th, 2025]
- Humatrope's Role in Treating Growth Retardation in American Males with CKD [Last Updated On: February 25th, 2025] [Originally Added On: February 25th, 2025]
- Exploring the Role of Humatrope in Managing Short Bowel Syndrome: A Comprehensive Review [Last Updated On: March 7th, 2025] [Originally Added On: March 7th, 2025]
- Exploring the Impact of Humatrope on Quality of Life in Men with Growth Hormone Deficiency [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Unveiling the Impact of Humatrope on Lipid Profiles in Growth Hormone Deficient American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Immunomodulatory Effects of Humatrope in Men with Growth Hormone Deficiency [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Impact of Humatrope on Carcinoid Syndrome in Growth Hormone Deficient Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Impact of Humatrope Therapy on Vision in American Males with Growth Hormone Deficiency [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Therapeutic Potential of Humatrope in Treating Sheehan's Syndrome: A Clinical Perspective [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Humatrope Therapy Enhances Sleep Patterns in American Men with Growth Hormone Deficiency [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Humatrope Enhances Muscle Strength in Growth Hormone Deficient American Males: A Review [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Humatrope Therapy: Enhancing Cardiovascular Health in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Humatrope: Enhancing Wound Healing in American Males - Clinical Insights and Future Prospects [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Humatrope: Enhancing Growth and Life Quality in American Males with Noonan Syndrome [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Humatrope's Impact on Cognitive Function in American Males with Growth Hormone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Humatrope's Impact on Insulin Sensitivity in Men with Growth Hormone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Humatrope's Role in Managing HIV-Associated Wasting in American Males [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Humatrope: A Key Therapy for Growth Deficiencies in SGA Infants [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Humatrope: Safe, Effective Growth Hormone Therapy for American Males with Growth Disorders [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Humatrope: Boosting Energy and Vitality in American Males with Growth Hormone Deficiency [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Humatrope's Impact on Metabolic Syndrome in American Males with GHD: Efficacy and Safety [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Humatrope's Role in Managing Cachexia Among American Male Cancer Patients [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope: Anti-Aging Potential and Risks for American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope's Impact on Hair Growth in American Men with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope Therapy Enhances Dental Development in American Boys with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope Therapy's Impact on Vision in Growth Hormone Deficient American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope's Potential in Managing Hyperthyroidism: Insights for American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Humatrope Therapy's Impact on Adrenal Function in Growth Hormone Deficient American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Humatrope Therapy's Impact on Renal Function in American Males with GHD [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Humatrope Enhances Immune Function in American Men with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Humatrope's Role in Managing Cystic Fibrosis: Focus on American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Humatrope: A Beacon of Hope for American Males with SHOX Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope Therapy: A Promising Treatment for Chronic Fatigue Syndrome in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Impact on Mental Health in American Men with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope: Managing Pituitary Tumors and Growth Hormone Deficiency in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Impact on Gastrointestinal Function in American Men with GHD [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Impact on Fertility in American Males with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Impact on Parathyroid Function in American Males with GHD [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Impact on Thyroid Function in American Males with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope Therapy for Growth Hormone Deficiency Post-Cranial Irradiation in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope: Enhancing Growth and Health in American Males with GH Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope: Enhancing Bone Health in Males with Osteoporosis and Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope Therapy Enhances Skin Health in American Males with GHD [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Humatrope's Potential Benefits for American Males with Chronic Liver Disease [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Humatrope Therapy and Adrenal Insufficiency in American Males with GHD: A Comprehensive Analysis [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Humatrope's Role in Managing Congenital Adrenal Hyperplasia in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Humatrope: A Novel Treatment for Hypoparathyroidism in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Humatrope's Potential in Treating Rheumatoid Arthritis in American Males: An Overview [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Humatrope Enhances Pulmonary Function in Men with Growth Hormone Deficiency: A Review [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Humatrope: Enhancing Diabetes Management in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Humatrope's Role in Treating Anorexia Nervosa Among American Males: Benefits and Considerations [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Humatrope Therapy Enhances Hypothalamic Function in American Males with GHD [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Humatrope Therapy: A Promising Approach for Hyperparathyroidism in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Humatrope's Role in Managing Hypergonadism: Insights for American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Humatrope's Impact on Hearing in American Males with Growth Hormone Deficiency [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Humatrope: A Promising Adjunct Therapy for Hypothyroidism in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Humatrope's Role in Managing Addison's Disease: Benefits for American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Humatrope's Impact on Gonadal Function in American Males with Growth Hormone Deficiency [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Humatrope: Treating Growth Hormone Deficiency and Cushing's Syndrome in American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Humatrope's Role in Managing Hypogonadism: Insights for American Males [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Humatrope's Role in Treating GHD and Conn's Syndrome in American Males [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Humatrope Therapy: A Novel Approach to Managing Pheochromocytoma [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Humatrope's Therapeutic Impact on Carcinoid Syndrome in American Males with GHD [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Humatrope's Impact on Gigantism in American Males with Growth Hormone Deficiency [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Humatrope Therapy: Managing GHD and Acromegaly in American Males [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Humatrope's Role in Enhancing Neuroendocrine Tumor Treatment and Quality of Life [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Humatrope's Role in Treating Craniopharyngioma in American Males: A Comprehensive Guide [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Humatrope's Potential in Managing Multiple Endocrine Neoplasia Syndromes: A Comprehensive Overview [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Humatrope as Adjunct Therapy for Prolactinoma: Mechanisms, Clinical Use, and Future Research [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Humatrope Therapy: A New Hope for American Males with Diabetes Insipidus [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Humatrope's Role in Managing Sheehan's Syndrome: Insights for American Males [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Humatrope: A Vital Treatment for Growth Hormone Deficiency in American Males Post-Pituitary Apoplexy [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Humatrope's Impact on Central Precocious Puberty in American Males with GHD [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Humatrope Therapy's Impact on Empty Sella Syndrome in American Males with GHD [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Humatrope's Impact on Lymphocytic Hypophysitis in Men with Growth Hormone Deficiency [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Humatrope's Role in Treating SIADH: A Focus on American Males [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Humatrope Therapy: Enhancing Growth and Development in American Males with Down Syndrome [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Humatrope's Impact on Growth Hormone Deficiency in American Males with CHARGE Syndrome [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Humatrope's Impact on Prader-Willi Syndrome in American Males: Growth, Health, and Quality of Life [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Humatrope's Impact on Kallmann Syndrome and Growth Hormone Deficiency in American Males [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]