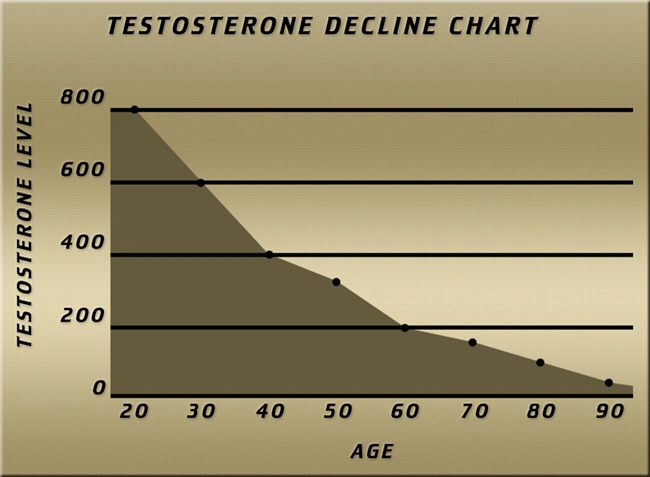
Introduction
Testosterone replacement therapy (TRT) has become increasingly prevalent among American males seeking to address symptoms of hypogonadism. Among the various modalities of TRT, Testim testosterone gel has gained popularity due to its ease of use and perceived efficacy. However, the long-term effects of this therapy on vital organ systems, particularly the kidneys, remain a subject of ongoing research and debate. This article delves into a nephrological study that meticulously monitored renal parameters in American males undergoing treatment with Testim testosterone gel, aiming to shed light on its impact on kidney function.
Study Design and Methodology
The study in question was a prospective, observational cohort analysis conducted over a 24-month period. It involved 250 American males aged 30 to 65 years who were prescribed Testim testosterone gel for clinically diagnosed hypogonadism. Participants were monitored at regular intervals, with renal function assessed through a battery of tests including serum creatinine, estimated glomerular filtration rate (eGFR), and urine analysis for proteinuria and microalbuminuria. The study aimed to detect any significant changes in these parameters that could be attributed to the use of Testim testosterone gel.
Baseline Renal Function and Patient Characteristics
At the outset of the study, the cohort's baseline renal function was within normal limits, with an average eGFR of 92 mL/min/1.73m². Participants were stratified based on age, body mass index (BMI), and pre-existing comorbidities such as hypertension and diabetes, which are known to influence renal function. This stratification allowed for a more nuanced analysis of the data, accounting for potential confounding variables.
Monitoring Renal Parameters Over Time
Throughout the 24-month study period, renal function parameters were closely monitored. Serum creatinine levels remained stable, with no statistically significant increase observed in the cohort. The eGFR, a more sensitive indicator of kidney function, also showed no significant decline over time. Urine analysis revealed no increase in proteinuria or microalbuminuria, suggesting that Testim testosterone gel did not induce glomerular damage or compromise the renal filtration barrier.
Subgroup Analysis and Risk Stratification
A subgroup analysis was performed to assess the impact of Testim testosterone gel on individuals with pre-existing risk factors for renal disease. In patients with hypertension or diabetes, no accelerated decline in renal function was observed compared to those without these conditions. This finding suggests that Testim testosterone gel may be safely used in American males with these common comorbidities, provided that they are closely monitored by healthcare professionals.
Potential Mechanisms and Clinical Implications
The absence of adverse renal effects observed in this study may be attributed to the pharmacokinetics of Testim testosterone gel, which is designed for transdermal absorption and gradual release. This delivery method may minimize the risk of sudden spikes in serum testosterone levels, which could potentially exert nephrotoxic effects. Clinically, these findings imply that Testim testosterone gel can be considered a viable option for TRT in American males, even in those with a higher baseline risk for renal disease.
Limitations and Future Directions
While this study provides valuable insights into the renal safety profile of Testim testosterone gel, it is not without limitations. The sample size, although substantial, may not be representative of the entire American male population. Additionally, the study duration of 24 months, while longer than many similar investigations, may not be sufficient to capture long-term renal effects. Future research should aim to include larger cohorts and extend the monitoring period to further validate these findings.
Conclusion
In conclusion, this nephrological study monitoring renal parameters in American males treated with Testim testosterone gel found no significant adverse effects on kidney function over a 24-month period. These results suggest that Testim testosterone gel may be a safe option for testosterone replacement therapy in this population, even among individuals with pre-existing risk factors for renal disease. However, ongoing vigilance and further research are warranted to ensure the long-term renal safety of this widely used TRT modality.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- 0001) Testim Gel: Enhancing Life Quality in Age-Related Testosterone Decline for American Men [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- 0002) Testim Testosterone Gel: Effective Low Testosterone Treatment for American Men [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- 0003) Testim Testosterone Gel: Efficacy, Application, and Side Effects for Hypogonadism Treatment [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- 0004) Testim Testosterone Gel: Enhancing Physical Performance in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- 0005) Testim Testosterone Gel: Benefits, Risks, and Usage for American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- 0006) Testim Testosterone Gel: Effective, Convenient TRT for American Men's Hypogonadism Management [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- 0007) Testim Testosterone Gel: Enhancing American Men's Health and Well-being [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- 0008) Testim Testosterone Gel: Long-Term Benefits and Risks for Men's Health [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- 0009) Testim Testosterone Gel: Benefits, Application, and Lifestyle Integration for American Men [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- 0010) Testim Gel: Boosting Testosterone Safely with Regular Monitoring [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- 0011) Testim Testosterone Gel: Enhancing Life for American Men with Low Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- 0012) Testim Gel: Restoring Vitality in American Men with Testosterone Deficiency [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- 0013) Testim Testosterone Gel: A Comprehensive Guide for American Men's Hormone Therapy [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- 0014) Testim Testosterone Gel: Enhancing Male Health in America [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- 0015) Testim Testosterone Gel: Enhancing Bone Density in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- 0016) Testim Testosterone Gel: Enhancing Vitality in American Men with Hypogonadism [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0017) Testim Testosterone Gel: Enhancing Male Health and Well-being in America [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0018) Testim Testosterone Gel: Enhancing Men's Health and Vitality in America [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0019) Testim Testosterone Gel: Revolutionizing Libido Enhancement for American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0020) Testim Testosterone Gel: Enhancing Muscle Mass and Health in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0021) Testim Gel: Enhancing American Men's Vitality Through Testosterone Replacement Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0022) Testim Testosterone Gel: Effects on Skin Health in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0023) Testim Testosterone Gel: Enhancing Male Vitality and Well-being in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0024) Testim Testosterone Gel: A New Era in Hormone Replacement for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0025) Testim Testosterone Gel: Combating Muscle Loss in Aging American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0026) Testim Testosterone Gel: Enhancing Cognitive Function in American Males with Low Testosterone [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0027) Testim Testosterone Gel Enhances Sleep Quality in American Males: Clinical Insights [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0028) Testim Testosterone Gel: Absorption, Effectiveness, and Safety for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0029) Testim Testosterone Gel: Enhancing Men's Sexual Health and Vitality in America [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0030) Testim Testosterone Gel: Enhancing Men's Health and Vitality in American Wellness Programs [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0031) Testim Gel: Enhancing American Men's Vitality with Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0032) Testim Testosterone Gel: Enhancing Libido, Strength, and Mood in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0033) Testim Gel: Enhancing Mood, Cognition, and Energy in American Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0034) Testim Testosterone Gel: Revolutionizing Male Health in America [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0035) Testim Testosterone Gel: Benefits and Usage for American Men with Hypogonadism [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0036) Managing Testim Testosterone Gel Side Effects in American Men: A Comprehensive Guide [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0037) Testim Testosterone Gel: Safety, Usage, and Risks for Low Testosterone Treatment [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0038) Testim Testosterone Gel: Revolutionizing Weight Management for American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0039) Testim Testosterone Gel: Combating Fatigue in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0040) Testim Testosterone Gel: Benefits and Guidelines for Diabetic Men with Low Testosterone [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0041) Testim Testosterone Gel: Effects on Hair Growth in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0042) Testim Testosterone Gel: Benefits and Risks for American Men with Heart Disease [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0043) Testim Testosterone Gel: Restoring Hormonal Balance in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0044) Testim Testosterone Gel: Enhancing Athletic Performance in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0045) Testim Gel: Effective Hypogonadism Treatment for American Men's Health and Vitality [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0046) Testim Testosterone Gel: A Promising Treatment for Mood Disorders in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0047) Testim Testosterone Gel: Enhancing Male Fertility in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- 0048) Testim Testosterone Gel: Enhancing Cardiovascular Health in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- 0049) Testim Testosterone Gel: Enhancing Stress Management in American Males with Low Testosterone [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- 0050) Testim Testosterone Gel: Enhancing Health for Obese Men with Low Testosterone [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- 0051) Testim Testosterone Gel: Enhancing Immune Function and Health in American Men [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- 0052) Testim Testosterone Gel: A Promising Solution for Chronic Fatigue in American Men [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- 0053) Testim Testosterone Gel: Enhancing Mental Health in American Men with Low Testosterone [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- 0054) Testim Testosterone Gel: A Promising Treatment for Osteoporosis in American Men [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- 0055) Testim Testosterone Gel: Impacts on Prostate Health and Monitoring Strategies [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- 0056) Testim Gel: Enhancing Life for American Men with Low Testosterone and Thyroid Issues [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- 0057) Testim Testosterone Gel: Potential Benefits for Men with Autoimmune Disorders [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- 0058) Testim Testosterone Gel: Managing Hypogonadism and Hypertension in American Men [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- 0059) Testim Testosterone Gel: Enhancing Post-Surgical Recovery in American Men [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- 0060) Testim Testosterone Gel: Enhancing Liver Function in American Men with Low Testosterone [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- 0061) Testim Testosterone Gel: Potential Benefits for Joint Health in American Males [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- 0062) Testim Testosterone Gel: Impact on Kidney Health in American Men with Hypogonadism [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- 0063) Testim Testosterone Gel: Efficacy and Safety for American Men with Allergies [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- 0064) Testim Testosterone Gel: Enhancing Life for Men with Sleep Apnea and Low Testosterone [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- 0065) Testim Testosterone Gel: A Promising Treatment for Anxiety in American Males [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- 0066) Testim Gel: A Promising Solution for Chronic Pain in American Men [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- 0067) Testim Testosterone Gel's Impact on Hearing in American Men: Recent Studies and Insights [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- 0068) Testim Testosterone Gel: Enhancing Digestive Health in American Men with Low Testosterone [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- 0069) Testim Testosterone Gel: Enhancing Respiratory Health in American Men [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- 0070) Testim Testosterone Gel: Enhancing Vision in American Males - Emerging Research and Applications [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- 0071) Testim Testosterone Gel: Managing Hypogonadism and Sensitive Skin in American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- 0072) Testim Testosterone Gel: Benefits and Considerations for American Men with Arthritis [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- 0073) Testim Testosterone Gel: Enhancing Life for American Men with Neurological Disorders [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- 0074) Testim Testosterone Gel: Enhancing Nail Strength in American Males [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- 0075) Testim Testosterone Gel: A Promising Treatment for Depression in American Males [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- 0076) Testim Gel: Enhancing Testosterone and GI Health in American Men [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- 0077) Testim Testosterone Gel: Enhancing Dental Health in American Men [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- 0078) Decade-Long Study Reveals Testim Gel Boosts Bone Density in Diverse American Males [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- 0079) Testim Testosterone Gel: Enhancing Male Vitality and Health in the U.S. [Last Updated On: April 23rd, 2025] [Originally Added On: April 23rd, 2025]
- 0080) Testim Testosterone Gel vs. Antidepressants: Mood Enhancement in American Males [Last Updated On: April 23rd, 2025] [Originally Added On: April 23rd, 2025]