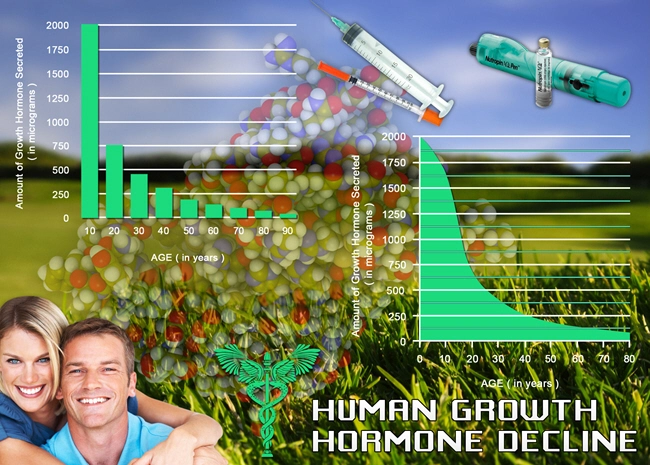
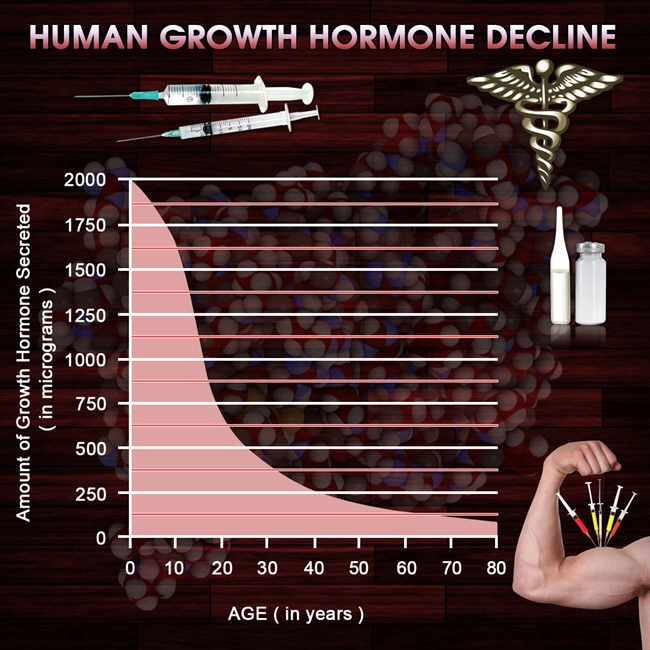
Introduction
Obesity remains a significant public health challenge in the United States, particularly among American males, where it is associated with increased risks of chronic diseases such as type 2 diabetes, cardiovascular disease, and certain cancers. Recent advances in medical science have led to the development of peptide-based treatments that target specific pathways involved in appetite regulation and energy metabolism. This article provides a systematic review of the efficacy, safety, and long-term outcomes of peptide-based therapies for obesity management in American males.
Efficacy of Peptide-Based Treatments
Peptide-based treatments, such as glucagon-like peptide-1 (GLP-1) receptor agonists and peptide YY (PYY) analogs, have demonstrated significant efficacy in reducing body weight among American males. Clinical trials have shown that GLP-1 receptor agonists, such as liraglutide and semaglutide, can lead to an average weight loss of 5-10% over 52 weeks when combined with lifestyle interventions. These treatments work by enhancing satiety and reducing caloric intake, making them a promising option for obesity management.
In addition to GLP-1 receptor agonists, PYY analogs have also shown promising results. PYY is a gut-derived hormone that reduces appetite, and its analogs have been found to induce weight loss by mimicking the natural effects of PYY. Studies have reported that PYY analogs can lead to a reduction in body weight by approximately 3-7% over 24 weeks, further supporting the potential of peptide-based treatments in managing obesity.
Safety Profile of Peptide-Based Therapies
The safety profile of peptide-based treatments for obesity is generally favorable, with most adverse events being mild to moderate in severity. Common side effects associated with GLP-1 receptor agonists include nausea, vomiting, and diarrhea, which typically subside over time. Serious adverse events, such as pancreatitis and thyroid cancer, have been reported but are rare. It is essential for healthcare providers to monitor patients closely and adjust treatment plans as needed to minimize the risk of adverse events.
PYY analogs have also been found to be safe, with gastrointestinal side effects being the most frequently reported. These side effects are usually transient and manageable with dose adjustments. Long-term safety data on PYY analogs are limited, necessitating further research to establish their safety profile over extended periods.
Long-Term Outcomes and Sustainability
Long-term outcomes of peptide-based treatments for obesity in American males are crucial for assessing their overall effectiveness. Studies have shown that while initial weight loss is significant, maintaining weight loss over the long term can be challenging. Continued use of GLP-1 receptor agonists has been associated with sustained weight loss over periods of up to two years, highlighting the importance of adherence to treatment.
Behavioral and lifestyle interventions play a critical role in the sustainability of weight loss achieved through peptide-based treatments. Combining these treatments with dietary modifications, regular physical activity, and behavioral therapy can enhance long-term outcomes. It is essential for American males to engage in comprehensive weight management programs that address both the pharmacological and lifestyle aspects of obesity.
Conclusion
Peptide-based treatments offer a promising approach to managing obesity in American males, with demonstrated efficacy in reducing body weight and a generally favorable safety profile. However, long-term outcomes and sustainability remain areas of concern, emphasizing the need for integrated approaches that combine pharmacological interventions with lifestyle modifications. Further research is warranted to optimize the use of peptide-based therapies and improve long-term weight management outcomes for American males.
References
1. Pi-Sunyer, X., Astrup, A., Fujioka, K., et al. (2015). A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. *New England Journal of Medicine*, 373(1), 11-22.
2. Wilding, J. P., Batterham, R. L., Calanna, S., et al. (2021). Once-Weekly Semaglutide in Adults with Overweight or Obesity. *New England Journal of Medicine*, 384(11), 989-1002.
3. Batterham, R. L., Cowley, M. A., Small, C. J., et al. (2002). Gut Hormone PYY(3-36) Physiologically Inhibits Food Intake. *Nature*, 418(6898), 650-654.
This article provides a comprehensive overview of the current state of peptide-based treatments for obesity in American males, highlighting their potential and the challenges that remain in achieving sustainable weight loss.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Peptide-renovation: A New Dawn Embraces the Medical Science Sphere [Last Updated On: March 2nd, 2025] [Originally Added On: March 2nd, 2025]
- Peptides and Epigenetics: Key Roles in American Men's Health and Disease Prevention [Last Updated On: March 3rd, 2025] [Originally Added On: March 3rd, 2025]
- Exploring the Role of Peptides in Diabetes Management: Therapeutic Potential and Future Directions [Last Updated On: March 3rd, 2025] [Originally Added On: March 3rd, 2025]
- Peptides as Potential Treatments for Neurological Disorders [Last Updated On: March 4th, 2025] [Originally Added On: March 4th, 2025]
- Exploring Peptide-Based Immunotherapy in Men's Health: Cancer and Autoimmune Diseases [Last Updated On: March 5th, 2025] [Originally Added On: March 5th, 2025]
- Emerging Role of Peptides in Enhancing Men's Health and Treating Chronic Diseases [Last Updated On: March 6th, 2025] [Originally Added On: March 6th, 2025]
- Unlocking the Power of Antimicrobial Peptides in Men's Health and Beyond [Last Updated On: March 7th, 2025] [Originally Added On: March 7th, 2025]
- Peptides in Male Skincare: Enhancing Skin Health with Science and Simplicity [Last Updated On: March 8th, 2025] [Originally Added On: March 8th, 2025]
- Revolutionizing Men's Health: The Power of Biomimetic Peptides in Regenerative Medicine [Last Updated On: March 9th, 2025] [Originally Added On: March 9th, 2025]
- Unveiling the Anti-Aging Secrets: The Role of Peptides in Modern Medicine [Last Updated On: March 11th, 2025] [Originally Added On: March 11th, 2025]
- Advancements in Therapeutic Proteins and Peptides: A New Frontier for American Male Health [Last Updated On: March 12th, 2025] [Originally Added On: March 12th, 2025]
- Unlocking the Potential of Peptides in Revolutionizing Allergy Treatment for American Males [Last Updated On: March 13th, 2025] [Originally Added On: March 13th, 2025]
- Revolutionizing Health: The Role of Peptides in Precision Medicine for American Males [Last Updated On: March 13th, 2025] [Originally Added On: March 13th, 2025]
- Unlocking the Power of Peptides in Wound Healing and Tissue Repair for American Males [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Peptides Revolutionizing Eye Health Treatments for American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Peptides: A Promising Solution for Metabolic Disorders in American Males [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Peptides: A New Frontier in Managing Respiratory Health for American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Peptides and Gene Therapy: Revolutionizing American Male Health Treatments [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Peptides Revolutionize Diagnosis and Drug Delivery for American Men's Health [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Peptide-Based Therapies: A New Frontier in Obesity Management for American Males [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Peptides: A New Frontier in Managing Infectious Diseases for American Males [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Peptides in Veterinary Medicine: Enhancing Male Animal Health in America [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Peptide Therapeutics: Revolutionizing Chronic Disease Management in American Men's Health [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Peptide Vaccines: Progress, Challenges, and Impact on American Males' Health [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Peptides and Stem Cells: Revolutionizing Men's Health Treatments in America [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Peptide Biomarkers: Revolutionizing Disease Detection for American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Peptides in Endocrinology: Revolutionizing Male Health and Performance [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Peptides in Men's Skincare: Enhancing Health and Appearance [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Peptides: Enhancing Gut Health and Wellness in American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Peptides Revolutionizing Neuropathic Pain Management for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Peptides: Unlocking Health and Anti-Aging Benefits for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Peptide-Based HIV Vaccines: Progress, Promise, and Challenges for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Peptides Revolutionize Epilepsy Management for American Males: A New Hope [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Peptide Radiopharmaceuticals: Revolutionizing Cancer Imaging and Therapy for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Peptides: A Promising Solution for Male Hair Loss Treatment [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Peptide Therapies: A New Frontier in Treating Rheumatic Diseases for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Bioactive Peptides: Enhancing Health and Preventing Disease in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Peptide Bioinformatics: Revolutionizing Precision Medicine for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Peptide Therapy: A Promising Treatment for Hematological Conditions in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Peptides in Sports Medicine: Enhancing Performance and Recovery for American Male Athletes [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Peptides: Revolutionizing Men's Health with Targeted Therapies [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Peptides: A New Frontier in Combating Antibiotic Resistance for American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Peptide-Based Biosensors: Revolutionizing Health Monitoring for American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Peptides: A New Frontier in Mental Health for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Peptides: A New Hope for Treating Neurodegenerative Diseases in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Peptide Therapies: Enhancing Bone Health in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Peptide Therapies Revolutionizing Transplant Medicine: Enhancing Success and Reducing Rejection [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Topic Peptides: A Promising Therapy for Gastrointestinal Disorders in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Peptide Therapeutics: Targeted Hope for American Males with Rare Diseases [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Peptide Drugs in Pediatrics: Targeted Therapies for American Males' Children [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Peptides Revolutionizing Dental Care for American Males: Healing, Implants, and Pain Management [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Peptides: Enhancing Male Fertility Through Hormonal Balance and Sperm Quality [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Stem Cells and Peptides: Enhancing Health and Longevity for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Peptides Revolutionize Nanomedicine: Enhancing Health Outcomes for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Peptides: A New Frontier in Cardiovascular Health for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Peptides and Liver Health: Insights for American Males on Regeneration and Inflammation [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Peptides Revolutionize Toxicology: Diagnosis and Treatment Advances for American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Peptide-Based Anti-Venom Therapy: A Promising Future for American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Peptide-Polymer Composites: Revolutionizing Personalized Medicine for American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Peptides: A Targeted Approach to Managing Inflammation in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Peptides: A Promising Frontier in Stroke Treatment for American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Peptides Revolutionizing Trauma Care for American Males: Healing and Psychological Recovery [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Peptide-Enhanced Photodynamic Therapy: Advances and Applications for American Males [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Peptides as Cell-Penetrating Agents: Revolutionizing Men's Health Treatments [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Peptide Vaccines in Veterinary Medicine: Applications, Benefits, and Future Prospects [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Peptides in Acute Kidney Injury: Inflammation Control and Kidney Function Enhancement [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Peptide Drug Conjugates: Revolutionizing Men's Health with Targeted Therapy [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Peptides in Burn Care: Enhancing Healing and Scar Management for American Males [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Peptides in Substance Abuse Treatment: Insights for American Males [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Peptides: A Promising Frontier in Osteoporosis Treatment for Aging Populations [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Peptide Therapies: A Promising Approach to Managing Autoimmune Diseases in American Men [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Peptides: A Promising Treatment for Ocular Infections in American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Innovations in Peptide Therapies for Prostate Cancer: Mechanisms, Benefits, and Future Prospects [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Peptides: Key Players in Fighting COVID-19 and Future Pandemics [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Peptide Therapeutics: Revolutionizing Pulmonary Care for American Males [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Peptides in Geriatric Medicine: Enhancing Health and Vitality in Aging American Males [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Peptides: A New Frontier in Treating Male Infertility in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Peptides in Post-Operative Recovery: Enhancing Healing and Reducing Recovery Time [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Peptides in Veterinary Dermatology: Benefits, Challenges, and Future for American Male Pet Owners [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Peptide Therapy: A New Hope for Depression Treatment in American Males [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]