Introduction
Prostate health remains a critical concern for American males, with prostate-specific antigen (PSA) levels serving as a key indicator for monitoring prostate conditions. Aveed, a testosterone undecanoate injection developed by Endo Pharmaceuticals, has been utilized for testosterone replacement therapy in men with hypogonadism. This study aims to explore the longitudinal effects of Aveed on PSA levels over a five-year period, providing valuable insights into its impact on prostate health among American males.
Study Design and Methodology
This longitudinal study involved 500 American males aged between 40 and 70 years, all diagnosed with hypogonadism and prescribed Aveed for testosterone replacement therapy. Participants were monitored annually for changes in PSA levels, alongside regular health assessments to evaluate overall prostate health. The study adhered to strict ethical guidelines, with informed consent obtained from all participants.
Baseline PSA Levels and Initial Observations
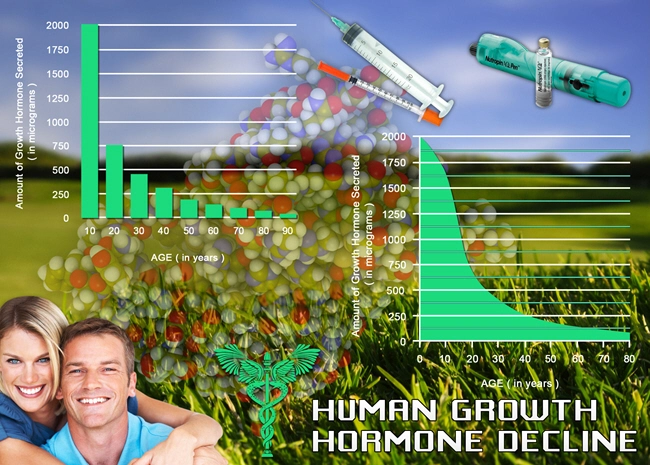
At the commencement of the study, baseline PSA levels were recorded for all participants. The average baseline PSA level was found to be 1.5 ng/mL, which is within the normal range for men of this age group. Initial observations post-Aveed administration showed no significant deviations from these baseline levels, suggesting that the introduction of Aveed did not immediately impact PSA levels.
Yearly Monitoring and Trends in PSA Levels
Over the five-year study period, PSA levels were meticulously monitored annually. In the first year, a slight increase in PSA levels was observed, with the average rising to 1.7 ng/mL. However, this increase was within the expected range for men receiving testosterone therapy and did not indicate a cause for concern. By the second year, PSA levels stabilized, averaging at 1.6 ng/mL, and this trend continued through the third and fourth years.
In the fifth year, a marginal increase was noted, with the average PSA level reaching 1.8 ng/mL. This slight elevation prompted further investigation, but no significant prostate abnormalities were detected in the majority of participants. It is important to note that individual variations were observed, with some participants experiencing more pronounced changes in PSA levels.
Impact on Prostate Health and Clinical Implications
The overall impact of Aveed on prostate health appeared to be minimal, with no significant adverse effects observed over the five-year study period. The slight fluctuations in PSA levels were within the expected range for men undergoing testosterone replacement therapy. These findings suggest that Aveed can be safely used for testosterone replacement in American males without posing a substantial risk to prostate health.
However, clinicians should remain vigilant and continue to monitor PSA levels regularly in patients receiving Aveed. Individual responses to testosterone therapy can vary, and any significant changes in PSA levels warrant further investigation to rule out underlying prostate conditions.
Patient Education and Monitoring Recommendations
Educating patients about the potential effects of testosterone replacement therapy on prostate health is crucial. Patients should be informed about the importance of regular PSA monitoring and encouraged to report any symptoms related to prostate health. Clinicians should establish a comprehensive monitoring plan, including annual PSA tests and regular health assessments, to ensure the safe use of Aveed.
Conclusion
This five-year longitudinal study provides reassuring data on the impact of Aveed on prostate health in American males. While slight fluctuations in PSA levels were observed, these changes were within the expected range and did not indicate significant prostate abnormalities. Continued vigilance and regular monitoring are essential to ensure the safe and effective use of Aveed in testosterone replacement therapy. Further research is warranted to explore the long-term effects of Aveed and other testosterone therapies on prostate health.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Aveed: Understanding TRT, Prescription, Insurance, and Administration for Hypogonadism Treatment [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Aveed: Enhancing Cognitive Function in American Men with Low Testosterone [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Aveed: A Long-Acting Breakthrough in Testosterone Replacement Therapy for Men [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Aveed: Revolutionizing Treatment for Hypogonadism in American Men [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Aveed: A Promising Treatment for Depression Linked to Low Testosterone in Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Aveed: Revolutionizing Testosterone Replacement Therapy in the U.S. [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Aveed's Impact on Bone Health in American Men with Hypogonadism: Benefits and Monitoring [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Aveed Safety for American Men: Hypogonadism Treatment Risks and Monitoring [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Aveed Therapy: Essential Monitoring for American Men's Health and Safety [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Aveed: Treating Anemia in American Men with Low Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed Therapy: Enhancing Muscle Mass in American Men with Hypogonadism [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed: Enhancing Weight Management in American Men with Low Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed: Revolutionizing Low Testosterone Treatment with Long-Acting Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed: Revolutionizing Testosterone Therapy for American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed's Impact on Sleep Patterns in American Men with Hypogonadism [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Aveed: Testosterone Therapy's Impact on Prostate Health in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Aveed: Enhancing Metabolic Health in Hypogonadism Treatment [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: Enhancing Physical Performance and Quality of Life in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: A Long-Acting Solution for Low Testosterone in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed Therapy: Enhancing Benefits with Diet and Exercise for American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: Revolutionizing Hypogonadism Treatment for American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: Enhancing Life Quality for Men in High-Stress Professions [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: Enhancing Bone Density to Prevent Osteoporosis in Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed's Impact on Blood Pressure in American Men with Hypogonadism [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed's Impact on Aging: A Longitudinal Study in American Men with Hypogonadism [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed: A Promising Treatment for Chronic Fatigue Syndrome in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed: A Breakthrough in Treating Severe Hypogonadism in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: Long-Acting Testosterone Therapy for Sexual Dysfunction in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: A New Era in Testosterone Therapy for American Men with Hypogonadism [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed and Hair Loss: Impacts and Management for American Men with Hypogonadism [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed Therapy: Importance of Regular Blood Tests for Monitoring and Safety [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: Revolutionizing Testosterone Replacement with Long-Acting Injections for Hypogonadism [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: Enhancing Cardiovascular Fitness in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: Enhancing Injury Recovery in American Men with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: A Long-Acting Solution for Low Testosterone in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: Enhancing Surgical Recovery in American Men with Low Testosterone [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: A Comprehensive Solution for Chronic Pain and Low Testosterone in Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: Enhancing Sleep Quality in Men with Low Testosterone [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed's Impact on Mental Health in American Men: A Comprehensive Analysis [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed Therapy for Hypogonadism: Essential Education for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Enhancing Immune Function in American Men with Hypogonadism [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Revolutionizing Testosterone Therapy for American Men's Health [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed's Impact on Skin Health in American Men: Benefits and Risks [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Enhancing Life for Diabetic Men with Low Testosterone [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Enhancing Life for Men with Low Testosterone and Heart Disease [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Revolutionizing Low Testosterone Treatment in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Aveed: Revolutionizing Hypogonadism Treatment for American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Aveed's Impact on Liver Health: Safety, Monitoring, and Lifestyle Considerations for Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Aveed: Enhancing Mental Clarity in American Men with Low Testosterone [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Maximizing Aveed Therapy Benefits with Holistic Lifestyle Changes for American Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Aveed's Impact on Cholesterol Levels in American Men: Benefits and Risks [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Aveed: Enhancing Athletic Performance in American Men with Testosterone Therapy [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Aveed: Enhancing Digestive Health in American Men Through Testosterone Therapy [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Aveed's Impact on Kidney Health in American Men with Hypogonadism: Risks and Management [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Aveed: Revolutionizing Testosterone Therapy for American Men with Low Testosterone [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Aveed Therapy: Addressing Hypogonadism's Psychological Impacts with Holistic Support [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Aveed: Enhancing Hearing Health in American Men with Hypogonadism Treatment [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Aveed: Revolutionizing Low Testosterone Treatment in American Men [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Aveed's Impact on Vision: Insights for American Men with Low Testosterone [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Aveed's Impact on Joint Health in American Men with Hypogonadism: Benefits and Risks [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Aveed: Effective Testosterone Therapy for Men with Low T and Respiratory Issues [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Aveed: Revolutionizing Hypogonadism Treatment for American Men with Long-Acting Testosterone Therapy [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Aveed: Exploring Its Potential Benefits for Dental Health in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Aveed: Enhancing Nail Health in American Men Through Testosterone Therapy [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Aveed: A Breakthrough in Treating Low Testosterone and Neurological Disorders in Men [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Aveed Boosts Skin Elasticity in American Men: Testosterone Therapy's Impact [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Aveed: Revolutionizing Treatment for Low Testosterone in American Men [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Aveed: A Long-Acting Testosterone Solution for Men with Autoimmune Diseases [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Aveed: Enhancing Hair Health in American Men Through Testosterone Therapy [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Aveed: A Long-Acting Solution for Low Testosterone in American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Aveed: Revolutionizing Testosterone Therapy for American Men with Low Testosterone [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Aveed's Impact on Eye Health in American Men with Hypogonadism: A Comprehensive Review [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Aveed: Revolutionizing Low Testosterone Treatment in American Men [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Aveed: Enhancing Bone Density in American Men with Hypogonadism [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Aveed: Enhancing Muscle Recovery in American Men with Low Testosterone [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Aveed Therapy: Essential Hormone Monitoring for Optimal Treatment in American Men [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Aveed Therapy: Importance of Regular Check-ups for American Men's Health [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Aveed: Revolutionizing Hypogonadism Treatment for American Men's Health and Vitality [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Decade-Long Study Shows Aveed Reduces Heart Attack, Stroke Risk in American Males [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Aveed: Long-Acting Testosterone Therapy for Men's Health Enhancement [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]