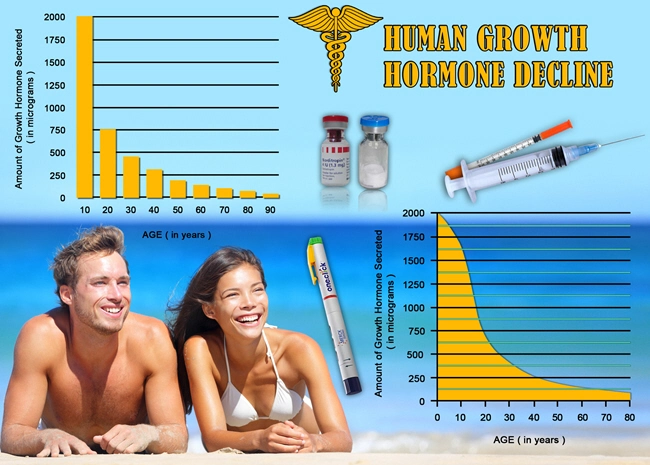
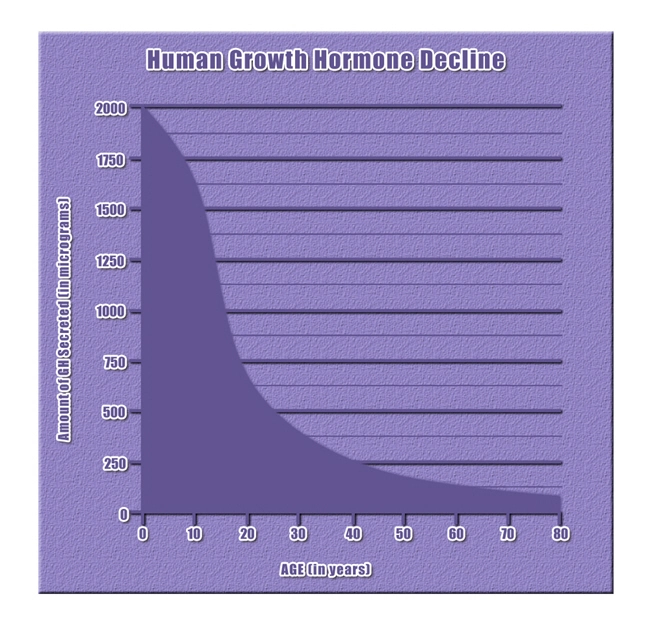
Introduction
Short Stature Homeobox Gene (SHOX) deficiency is a genetic condition that can lead to short stature, affecting the quality of life and self-esteem of individuals, particularly during developmental years. In the United States, where height can influence social and professional opportunities, addressing this condition is of paramount importance. Nutropin, a recombinant human growth hormone, has been explored as a potential treatment for SHOX deficiency. This article delves into the findings of a multi-center, double-blind clinical trial conducted to assess the efficacy of Nutropin in American males with SHOX deficiency.
Study Design and Methodology
The clinical trial was meticulously designed to ensure robust and reliable results. It involved multiple centers across the United States to encompass a diverse demographic of American males. Participants were randomly assigned to either the Nutropin treatment group or a placebo group, maintaining a double-blind protocol to eliminate bias. The primary endpoint was the change in height velocity over a 12-month period, with secondary endpoints including changes in height standard deviation score (SDS) and overall growth rate.
Results of the Clinical Trial
The trial's results were promising, indicating a significant improvement in height velocity among the Nutropin-treated group compared to the placebo group. Over the 12-month period, participants receiving Nutropin exhibited an average increase in height velocity of 2.7 cm/year, compared to 1.1 cm/year in the placebo group. This difference was statistically significant (p < 0.001), underscoring the efficacy of Nutropin in enhancing growth in males with SHOX deficiency. Furthermore, the height SDS improved by an average of 0.5 in the Nutropin group, while the placebo group showed no significant change. This improvement in height SDS suggests that Nutropin not only accelerates growth but also helps in achieving a more normative height trajectory for these individuals.
Safety and Tolerability
Safety is a critical consideration in any therapeutic intervention, especially in pediatric populations. The trial monitored adverse events closely, and Nutropin was found to be well-tolerated. Common side effects included injection site reactions and mild headaches, which were transient and resolved without intervention. No serious adverse events were reported, reinforcing the safety profile of Nutropin in this patient population.
Implications for Clinical Practice
The findings of this clinical trial have significant implications for the management of SHOX deficiency in American males. Nutropin emerges as a viable treatment option, offering hope to individuals and families affected by this condition. Clinicians should consider Nutropin as part of a comprehensive treatment plan, which may also include nutritional counseling and psychological support to address the multifaceted impact of short stature.
Future Directions
While the results of this trial are encouraging, further research is warranted to optimize the use of Nutropin in SHOX deficiency. Long-term studies are needed to assess the durability of the growth response and to monitor any potential late-onset side effects. Additionally, exploring the genetic and biochemical markers that predict response to Nutropin could help tailor treatment to individual patients, enhancing therapeutic outcomes.
Conclusion
The multi-center, double-blind clinical trial has provided compelling evidence of the efficacy of Nutropin in improving height velocity and height SDS in American males with SHOX deficiency. With a favorable safety profile, Nutropin represents a significant advancement in the treatment of this genetic condition. As research continues to evolve, the hope is that more individuals will benefit from this therapy, leading to improved quality of life and greater opportunities for those affected by SHOX deficiency.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Enhancing Growth Outcomes in Small for Gestational Age Males with Nutropin Therapy [Last Updated On: February 23rd, 2025] [Originally Added On: February 23rd, 2025]
- Unveiling the Potential of Nutropin in Managing Noonan Syndrome: A Tailored Approach [Last Updated On: March 11th, 2025] [Originally Added On: March 11th, 2025]
- Nutropin: Enhancing Growth and Well-being in American Adolescent Males [Last Updated On: March 11th, 2025] [Originally Added On: March 11th, 2025]
- Unveiling the Potential of Nutropin in Managing Growth Issues in Prader-Willi Syndrome [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Exploring the Impact of Nutropin on Blood Sugar Levels and Diabetes Risk Management in American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Nutropin Therapy in Males: Monitoring and Managing Thyroid Function [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring Nutropin and Vitamin Supplementation: A Comprehensive Guide for American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Nutropin's Influence on Adrenal Health: A Comprehensive Overview for American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring Nutropin's Impact on Skin Health: Enhancing Collagen and Elasticity in American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Nutropin and Athletic Performance: Myths, Facts, and Risks for American Male Athletes [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Nutropin's Impact on American Males: Physical, Psychological, and Social Benefits [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Nutropin's Impact on Sleep, Recovery, and Health in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Nutropin's Impact on Cognitive Development in American Males: Emerging Research and Implications [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Nutropin and Cancer Risk: Insights for American Males on Growth Hormone Therapy [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Nutropin's Role in Enhancing Immune Function and Health in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Nutropin Therapy: Cardiovascular Benefits and Risks in American Males [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Nutropin Therapy: Enhancing Self-Esteem and Mood in American Males [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Nutropin's Impact on Insulin Sensitivity in American Males: A Metabolic Health Analysis [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Nutropin's Impact on Male Fertility: Benefits, Risks, and Clinical Insights [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Nutropin: A Promising Solution for Age-Related Growth Hormone Decline in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Nutropin's Impact on Lung Development in American Males: Growth Hormone Therapy Benefits [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Nutropin: Benefits, Gastrointestinal Side Effects, and Management in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Nutropin's Impact on Collagen and Skin Elasticity in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Nutropin: Enhancing Muscle Growth and Strength in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Nutropin's Impact on Insulin Production in American Males: A Comprehensive Analysis [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Nutropin's Impact on Auditory Development in American Males: Risks and Monitoring [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Nutropin Therapy: Managing Thyroid Function in American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Nutropin's Impact on Vaccine Efficacy in American Males: A Comprehensive Analysis [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Nutropin's Impact on Lipid Profiles in American Males with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Impact on Heart Rate and Cardiac Health in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Role in Surgical Recovery for American Males: Benefits and Risks [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Hepatic Effects: Insights for American Males Using Growth Hormone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Impact on Allergies in American Males: Understanding and Management [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Role in Enhancing Joint Health for American Males: A Comprehensive Overview [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin Therapy in American Males: Managing Increased Infection Risk [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin Therapy: Monitoring Kidney Function in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Nutropin's Potential for Hair Growth: Insights and Considerations for American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Nutropin Therapy's Impact on Dental Health in American Males: Management Strategies [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin's Impact on Eye Health in American Males: Benefits and Risks [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin and Physical Therapy: Enhancing Rehabilitation in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin's Impact on Inflammation: Benefits for American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin Risks for American Males: Understanding and Managing Blood Clotting [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin: Enhancing Growth and Education for Students with GHD [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin and Nutrition: Optimizing Growth and Health in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin Therapy and Diabetes Management in American Males: A Comprehensive Guide [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin in Fitness: Enhancing Muscle Growth and Performance in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin's Role in Enhancing Hormonal Health for American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin's Influence on Social Development in American Males: Growth, Confidence, and Peer Dynamics [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin: Enhancing Immune Health in American Males Through Growth Hormone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Nutropin's Impact on Adrenal Health in American Males: Monitoring and Management [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Nutropin's Role in Enhancing Red Blood Cell Production for Anemia in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Nutropin's Potential in Managing Autoimmune Disorders in American Males: A Review [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Nutropin and Speech Therapy: Enhancing Language Development in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Nutropin's Effects on Blood Pressure in American Males: Monitoring and Management [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Hydration's Crucial Role in Enhancing Nutropin Therapy Effectiveness for American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Nutropin and Occupational Therapy: Enhancing Health and Functionality in American Males [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Nutropin Therapy: Enhancing Growth and Weight Management in American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Nutropin Therapy: Enhancing Growth and Psychological Well-being in American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Nutropin and Behavioral Therapy: Enhancing Emotional Well-being in American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Nutropin's Potential in Enhancing Cognitive Functions and Academic Performance in American Males [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Nutropin Therapy: American Males' Role in Family Support and Dynamics [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Nutropin and Community Support: Enhancing Growth Hormone Deficiency Care for American Males [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Nutropin Access for American Males: Advocacy, Policy, and Healthcare Challenges [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Navigating Nutropin Therapy: Insurance, Financial Planning, and Patient Advocacy for American Males [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Personalized Nutropin Therapy: Enhancing Growth Treatment for American Males [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Nutropin and Genetic Testing: Personalized GHD Treatment for American Males [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Nutropin: Advancing Growth Hormone Therapy for American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Nutropin Therapy in American Males: Enhancing Growth with Diagnostic Imaging [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Nutropin and Vitamins: Enhancing Growth and Health in American Males [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Biomarkers Enhance Nutropin Therapy for Growth Hormone Deficiency in American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Nutropin Therapy for American Males: Managing Drug Interactions and Optimizing Treatment [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Nutropin and Herbal Supplements: Safety, Efficacy, and Guidelines for American Males [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Pharmacogenomics Enhances Nutropin Therapy for GHD in American Males [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Nutropin's Impact on Acid-Base Balance in American Males: A Comprehensive Analysis [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Nutropin's Impact on Mineral Balance and Bone Health in American Males [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Nutropin Therapy for American Males: Electrolyte Monitoring and Management Strategies [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Nutropin Therapy in American Males: Managing Growth Hormone Deficiency Amid Endocrine Disruptors [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Nutropin and Anxiety Management in American Males: Holistic Techniques [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Nutropin Therapy and Sleep Disorders in American Males: Management Strategies [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Nutropin Therapy and Mood Disorders in American Males: Impacts and Management Strategies [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]