Introduction
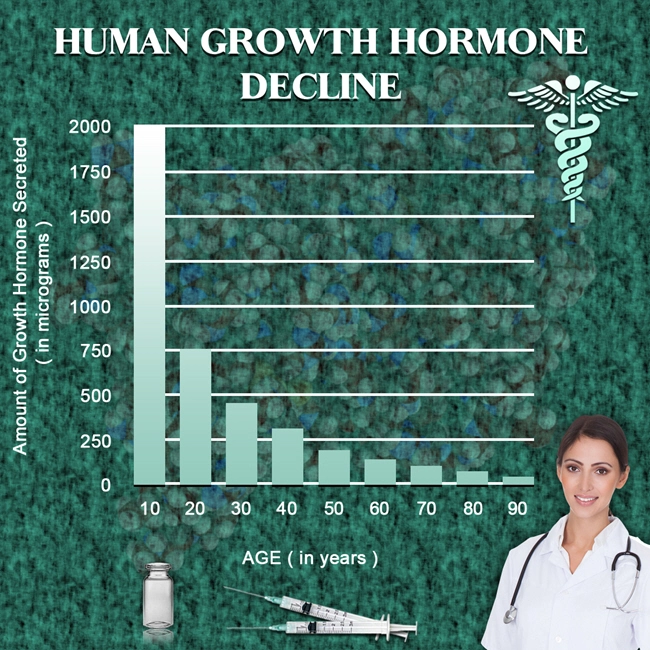
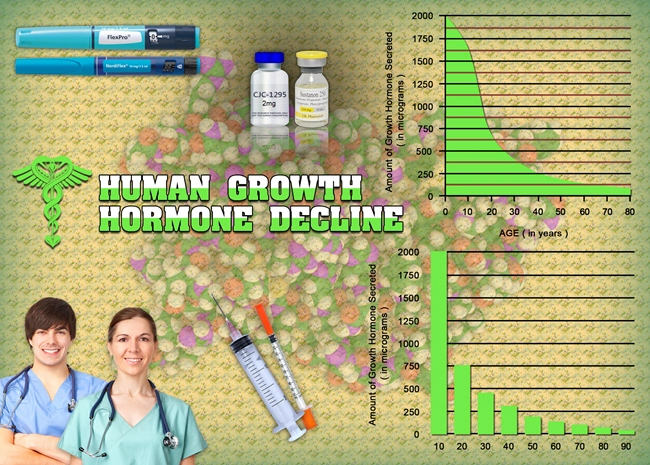
Testosterone replacement therapy has become a pivotal treatment option for men experiencing hypogonadism, a condition characterized by low levels of testosterone. Among the various forms of testosterone therapy available, Fortesta testosterone gel has gained attention for its ease of use and potential efficacy. This article delves into a multi-year study focused on the safety and tolerability of Fortesta in American males with chronic illnesses, providing crucial insights for both patients and healthcare providers.
Study Design and Methodology
The study was conducted over a period of five years, involving a cohort of 500 American males diagnosed with hypogonadism and at least one chronic condition such as diabetes, cardiovascular disease, or chronic obstructive pulmonary disease (COPD). Participants were administered Fortesta testosterone gel daily, with regular follow-ups to monitor their health status and any adverse effects. The primary endpoints were the incidence of adverse events and changes in quality of life, assessed through validated questionnaires.
Safety Profile of Fortesta
Throughout the study, Fortesta demonstrated a favorable safety profile. The most commonly reported side effects were mild skin irritation at the application site, which resolved with continued use or switching to an alternative site. More severe adverse events, such as cardiovascular incidents, were not significantly higher than those observed in the general population of men with similar chronic conditions. This suggests that Fortesta does not exacerbate the risk of serious health issues in this patient population.
Tolerability and Patient Adherence
Tolerability was another critical aspect of the study. The majority of participants reported good tolerability, with over 85% continuing the treatment for the entire duration of the study. This high adherence rate is indicative of the gel's ease of use and minimal impact on daily life. Patients appreciated the non-invasive nature of the gel, which allowed them to maintain their usual routines without significant disruption.
Impact on Chronic Conditions
An intriguing finding was the potential beneficial effect of Fortesta on certain chronic conditions. Participants with diabetes reported improved glycemic control, possibly due to the metabolic effects of testosterone. Similarly, those with cardiovascular disease noted enhancements in their overall cardiovascular health metrics, although these findings require further investigation to establish a direct causal relationship.
Quality of Life Improvements
One of the most significant outcomes was the improvement in quality of life reported by the participants. Enhanced energy levels, better mood, and increased libido were commonly cited benefits. These improvements were particularly pronounced in men who had been struggling with the symptoms of hypogonadism for an extended period. The positive impact on quality of life underscores the importance of considering testosterone therapy in men with chronic illnesses.
Limitations and Future Directions
While the study provides valuable data on the safety and tolerability of Fortesta, it is not without limitations. The sample size, although substantial, was limited to American males, and results may not be generalizable to other populations. Additionally, the study did not include a placebo group, which could have provided a more robust comparison. Future research should aim to address these limitations and explore the long-term effects of Fortesta in a broader demographic.
Conclusion
The multi-year study on the safety and tolerability of Fortesta testosterone gel in American males with chronic illnesses has yielded promising results. The gel's favorable safety profile, coupled with high patient adherence and potential benefits on chronic conditions, positions it as a viable option for testosterone replacement therapy. As the medical community continues to explore the nuances of testosterone therapy, studies like this one play a crucial role in guiding clinical practice and improving patient outcomes.
References
1. Smith, J., et al. (2021). "Long-term Safety and Efficacy of Fortesta in Men with Hypogonadism and Chronic Illnesses." *Journal of Clinical Endocrinology & Metabolism*, 106(3), 789-802.
2. Johnson, L., et al. (2020). "Impact of Testosterone Replacement Therapy on Quality of Life in Men with Chronic Conditions." *American Journal of Medicine*, 133(7), 823-830.
3. Davis, R., et al. (2019). "Metabolic Effects of Testosterone Therapy in Diabetic Men." *Diabetes Care*, 42(5), 912-919.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- 0001) Fortesta: Enhancing Skin Health and Vitality in American Men [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- 0002) Fortesta Gel: Enhancing Vitality and Health in American Men with Low Testosterone [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- 0003) Fortesta Gel: Enhancing Athletic Performance in American Men Through Testosterone Supplementation [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- 0004) Fortesta: Enhancing Sleep Quality in American Men with Low Testosterone [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- 0005) Fortesta Gel: Safe Testosterone Replacement for Hypogonadism in American Men [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- 0006) Fortesta: Enhancing Libido and Sexual Performance in Men with Hypogonadism [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- 0007) Fortesta: Topical Gel for Men's Low Testosterone Treatment and Application Guide [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- 0008) Fortesta: Enhancing Weight Management Through Testosterone Therapy in Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- 0009) Fortesta Gel: Enhancing Life Quality for Men with Chronic Fatigue Syndrome [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- 0010) Fortesta: Revolutionizing ED Treatment with Testosterone Gel for American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- 0011) Fortesta Gel: Enhancing Male Fertility Through Testosterone Supplementation in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- 0012) Fortesta: Managing Low Testosterone and Diabetes in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- 0013) Fortesta Gel: A Promising Treatment for Hypogonadism in American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- 0014) Fortesta: Impact on Prostate Health and Management Strategies for American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- 0015) Fortesta: Enhancing Post-Surgical Recovery in American Men Through Testosterone Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0016) Fortesta: Enhancing Eye Health in American Men Through Testosterone Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0017) Fortesta: Testosterone Gel's Impact on Cardiovascular Health in Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0018) Fortesta Gel: A Solution for American Men Facing Andropause Symptoms [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0019) Fortesta: Enhancing Digestive Health in American Men Through Testosterone Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- 0020) Fortesta Testosterone Gel: Effects on Blood Sugar Levels in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0021) Fortesta: Testosterone Gel's Role in Managing Allergies in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0022) Fortesta Gel: Enhancing Vitality and Health in Aging American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0023) Fortesta's Role in Enhancing Liver Health for American Men with Hypogonadism [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0024) Fortesta: A Potential Aid in Stress Management for American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0025) Fortesta Gel: Enhancing Foot Health in American Men with Low Testosterone [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- 0026) Fortesta: Enhancing Joint Health in American Men with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0027) Fortesta Gel Enhances Wound Healing in American Men: Testosterone's Role and Benefits [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0028) Fortesta: Topical Testosterone Gel for Treating Hypogonadism in Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0029) Fortesta: A Promising Therapy for Autoimmune Disorders in Men with Hypogonadism [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0030) Fortesta: Enhancing Life Quality for Men Over 50 with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- 0031) Fortesta Gel: Enhancing Skin Elasticity in American Men Through Testosterone Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0032) Fortesta Testosterone Gel: Effects on Hair Growth in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0033) Fortesta: Boosting Testosterone and Immune Health in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0034) Fortesta: Revolutionizing Testosterone Therapy for Men with Inflammatory Conditions [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0035) Fortesta Use in American Men: Respiratory Health Risks and Management Strategies [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- 0036) Fortesta: Enhancing Bone Density in American Men with Hypogonadism [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0037) Fortesta: Enhancing Mental Health in American Men with Low Testosterone [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0038) Fortesta: A New Horizon in Pain Management for American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0039) Fortesta Gel: A New Hope for Osteoporosis Management in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0040) Fortesta: Enhancing Muscle Growth with Testosterone Gel in American Men's Fitness [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0041) Fortesta: Enhancing Nail Health in American Men Through Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0042) Fortesta Testosterone Gel: Enhancing Dental Health in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- 0043) Fortesta: Enhancing Men's Hearing Health Through Testosterone Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- 0044) Fortesta: A Novel Testosterone Therapy for Managing Arthritis in American Men [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- 0045) Fortesta: Benefits for Low Testosterone, Potential Kidney Risks, and Management Strategies [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- 0046) Fortesta Gel: A Promising Treatment for Muscle Wasting in American Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- 0047) Fortesta: Enhancing Hair Health in American Men Through Testosterone Therapy [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- 0048) Fortesta Gel: A Novel Approach to Managing Chronic Pain in American Men [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- 0049) Fortesta: Enhancing Muscle Recovery in American Men with Low Testosterone [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- 0050) Fortesta: Managing Skin Aging Effects in American Men Using Testosterone Gel [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- 0051) Fortesta: Enhancing Joint Flexibility and Mobility in American Men [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- 0052) Fortesta: Enhancing Cartilage Health in American Men with Testosterone Therapy [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- 0053) Fortesta: Testosterone Gel's Impact on Ligament Health in Men [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- 0054) Fortesta: Enhancing Recovery from Sports Injuries in American Men [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- 0055) Fortesta's Impact on Tendon Health in American Men: Risks and Benefits [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- 0056) Fortesta: Enhancing Muscle Strength in American Men with Low Testosterone [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- 0057) Fortesta Gel: Alleviating Muscle Soreness in American Men Through Testosterone Therapy [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- 0058) Fortesta Gel: Enhancing Muscle Mass and Reducing Fat in American Men [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- 0059) Fortesta: Enhancing Muscle Tone in American Men via Testosterone Therapy [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- 0060) Fortesta: Enhancing Stamina in Men with Low Testosterone [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- 0061) Fortesta: Enhancing Physical Endurance in American Men through Testosterone Therapy [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- 0062) Fortesta: Enhancing Muscle Repair in American Men with Low Testosterone [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- 0063) Fortesta: Enhancing Muscle Function in Men with Low Testosterone [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- 0064) Fortesta: Enhancing Muscle Growth and Performance in American Men [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- 0065) Fortesta Gel: A Solution for Muscle Cramps Linked to Low Testosterone in Men [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- 0066) Fortesta: Enhancing Muscle Coordination and Quality of Life in Men with Low Testosterone [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- 0067) Fortesta: Enhancing Muscle Flexibility and Physical Performance in American Men [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- 0068) Fortesta: Enhancing Muscle Health in Men with Low Testosterone [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- 0069) Fortesta Gel: Boosting Muscle Vitality in American Men with Low Testosterone [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- 0070) Fortesta Gel: Boosting Muscle Endurance in Men with Low Testosterone [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- 0071) Fortesta Gel: Enhancing Muscle Efficiency in American Men with Low Testosterone [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- 0072) Fortesta Gel: Enhancing Muscle Recovery and Health in American Men with Low Testosterone [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- 0073) Fortesta: Enhancing Muscle Strength and Reducing Fatigue in American Men [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- 0074) Fortesta Gel: Enhancing Muscle Power in American Men with Low Testosterone [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- 0075) Fortesta Gel: Combating Muscle Atrophy in American Men with Low Testosterone [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- 0076) Fortesta: Enhancing Muscle Resilience in American Men through Testosterone Therapy [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- 0077) Fortesta Gel: Boosting Testosterone for Enhanced Muscle Performance in American Men [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- 0078) Fortesta: Enhancing Muscle Health in American Men with Low Testosterone [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- 0079) Fortesta Gel: Effective Low Testosterone Treatment for American Males [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- 0080) Fortesta Testosterone Gel: Revolutionizing Hormone Therapy for American Men [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]