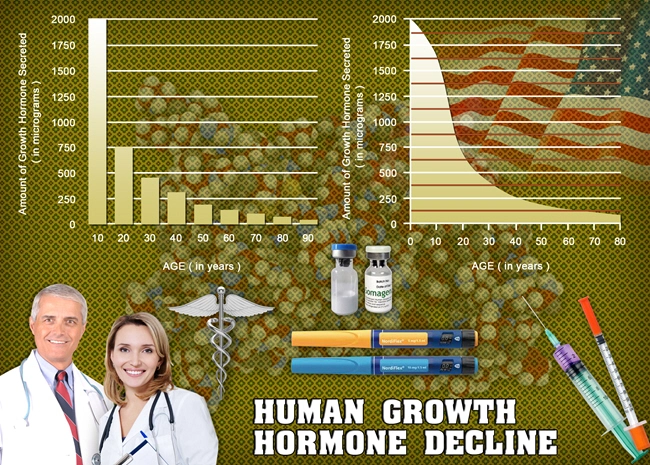
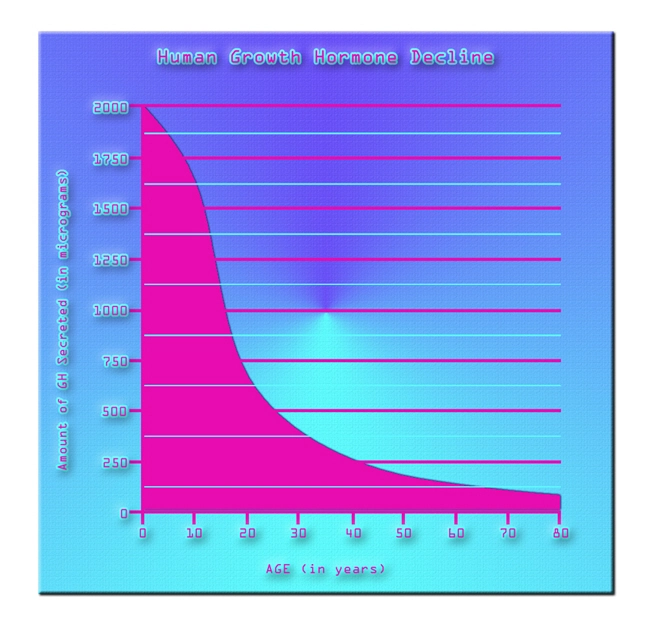
Introduction
Omnitrope, a recombinant human growth hormone, has been widely used for various medical conditions, including growth hormone deficiency and Turner syndrome. Despite its therapeutic benefits, concerns have been raised regarding its potential impact on liver function. This article presents a comprehensive retrospective analysis of liver enzyme levels over a decade in American males treated with Omnitrope, providing valuable insights into the drug's safety profile.
Study Design and Methodology
This retrospective study analyzed data from American males aged 18 to 65 who received Omnitrope treatment between 2010 and 2020. Liver function was assessed by monitoring levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT) at baseline and at regular intervals throughout the treatment period. The study population was stratified based on age, duration of treatment, and dosage to identify potential risk factors associated with liver enzyme elevations.
Results: Liver Enzyme Levels Over Time
The analysis revealed that the majority of American males treated with Omnitrope maintained stable liver enzyme levels throughout the study period. At baseline, the mean ALT, AST, and GGT levels were within the normal range for all age groups. Over the ten-year follow-up, only a small percentage of patients (less than 5%) experienced transient elevations in liver enzymes, which resolved without intervention or dosage adjustment.
Impact of Age and Treatment Duration
Further stratification of the data by age and treatment duration showed no significant correlation between these factors and liver enzyme levels. American males across all age groups and those receiving Omnitrope for varying durations demonstrated similar patterns of stable liver function. This finding suggests that the impact of Omnitrope on liver enzymes is not influenced by age or the length of treatment.
Dosage and Liver Enzyme Elevations
The study also investigated the potential relationship between Omnitrope dosage and liver enzyme elevations. Patients were categorized into low, medium, and high dosage groups based on their prescribed regimen. The analysis revealed no significant differences in liver enzyme levels among the dosage groups, indicating that Omnitrope dosage does not appear to be a risk factor for liver enzyme elevations in American males.
Clinical Implications and Safety Profile
The results of this decade-long study provide reassuring evidence regarding the safety of Omnitrope in relation to liver function in American males. The low incidence of transient liver enzyme elevations and the absence of significant risk factors suggest that Omnitrope can be safely used for the treatment of growth hormone deficiency and other approved indications. However, healthcare providers should continue to monitor liver function in patients receiving Omnitrope, as with any medication, to ensure early detection and management of potential adverse effects.
Limitations and Future Research
While this study provides valuable insights into the impact of Omnitrope on liver function, it is not without limitations. The retrospective nature of the analysis and the reliance on existing medical records may introduce potential biases. Future prospective studies with larger sample sizes and more comprehensive liver function assessments could further validate these findings and provide additional information on the long-term safety of Omnitrope in American males.
Conclusion
In conclusion, this retrospective analysis of liver enzyme levels over ten years in American males treated with Omnitrope demonstrates a favorable safety profile with regard to liver function. The majority of patients maintained stable liver enzyme levels, and only a small percentage experienced transient elevations that resolved without intervention. These findings support the continued use of Omnitrope for approved indications while emphasizing the importance of regular monitoring of liver function in patients receiving this medication.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Unveiling the Cardiovascular Benefits of Omnitrope in Growth Hormone Deficient American Males [Last Updated On: February 21st, 2025] [Originally Added On: February 21st, 2025]
- Omnitrope: Guide for American Males on Usage, Risks, and Monitoring [Last Updated On: February 22nd, 2025] [Originally Added On: February 22nd, 2025]
- Exploring the Impact of Omnitrope on Cognitive Development in Children: A Comprehensive Medical Analysis [Last Updated On: March 11th, 2025] [Originally Added On: March 11th, 2025]
- Unveiling the Journey of Omnitrope: From Manufacturing to Medical Application [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Unveiling the Potential of Omnitrope in Addressing Pediatric Growth Disorders Among American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Therapeutic Potential of Omnitrope in Pediatric Inflammatory Bowel Disease Management [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Therapeutic Potential of Omnitrope in Managing Noonan Syndrome [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Dermatological Benefits of Omnitrope in Growth Hormone Deficient American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Therapeutic Potential of Omnitrope in Managing Growth Hormone Deficiency Among American Males with Epilepsy [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Omnitrope: A Hopeful Solution for Growth Disorders in American Boys [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Omnitrope Enhances Insulin Sensitivity in American Males with Growth Hormone Deficiency [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Omnitrope's Role in Managing Lipid Profiles in American Men with GHD [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Omnitrope Therapy Enhances Muscle Strength in American Adult Males with GHD [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Omnitrope Therapy: Effects on Bone Age and Growth in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Omnitrope: Enhancing Growth and Quality of Life in Noonan Syndrome Patients [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Omnitrope for Adolescent Growth: Indications, Dosage, Monitoring, and Psychosocial Impact [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Omnitrope: Enhancing Growth and Life Quality in American Males with Idiopathic Short Stature [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Omnitrope: Enhancing Growth in SGA Infants - Mechanism, Efficacy, and Safety [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Optimizing Omnitrope Therapy for Growth Hormone Deficiency in American Males [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Omnitrope: Enhancing Final Height in American Boys with Growth Hormone Deficiency [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Omnitrope: Advancing Regenerative Medicine for American Males [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Omnitrope: Enhancing Growth and Quality of Life in IUGR Children in the US [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Omnitrope: Enhancing Health and Quality of Life in American Males with GHD [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Omnitrope Therapy: Enhancing Growth in American Male Cancer Survivors with GHD [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Omnitrope: Enhancing Growth in Children with Chronic Illnesses [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Omnitrope's Role in Managing Hypopituitarism for American Males: Efficacy and Considerations [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Omnitrope Therapy Enhances Sleep Quality in American Males with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Omnitrope: A Key Therapy for Growth Hormone Deficiency and Thyroid Disorders in Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope Enhances Immune Function in Growth Hormone Deficient American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope: Treating Growth Hormone Deficiency and Obesity in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope's Impact on Liver Function in American Males with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope: Effective GHD Treatment in American Males with Autoimmune Diseases [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope: Enhancing Quality of Life in HIV Patients with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope Therapy in Children: Monitoring Renal Function and Long-term Effects [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope's Efficacy in Treating GHD in American Males with Epilepsy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope: Enhancing Life for American Males with Short Bowel Syndrome [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Omnitrope: Treating Growth Hormone Deficiency and Rheumatoid Arthritis in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Omnitrope: Enhancing Growth and Life Quality in American Males with Hemophilia and GHD [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Omnitrope: Enhancing Growth and Quality of Life in Down Syndrome GHD [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Omnitrope Enhances Psychological Well-being in Men with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Omnitrope's Impact on GHD in American Males with Multiple Sclerosis: Benefits and Considerations [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope: Enhancing Bone Health in American Males with GHD and Osteoporosis [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope: Enhancing Growth in American Boys with Cystic Fibrosis [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope Therapy: Enhancing Growth and Eye Health in Children [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope's Impact on Respiratory Function in American Males with GHD [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope: A Tailored Treatment for GHD and Diabetes in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope's Impact on Hematological Parameters in American Males with GHD [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope's Efficacy in Enhancing Growth in American Males with Congenital Heart Disease [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope Therapy Enhances Gastrointestinal Health in American Male Children [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope: Enhancing Neurological Function in Growth Hormone Deficient Patients [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope's Role in Treating GHD in American Males with Sickle Cell Disease [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope's Role in Treating PCOS-Related GHD: Benefits for American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope: A Tailored Treatment for GHD in Asthmatic American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope Therapy's Impact on Skin Health in Pediatric Patients: A Comprehensive Review [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope's Potential in Managing Autism Spectrum Disorders: Insights for American Families [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Omnitrope Therapy's Impact on Auditory Health in Pediatric Patients: Insights for American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Omnitrope Therapy: Impacts on Dental Health in Children [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Omnitrope: A Potential Treatment for Growth Hormone Deficiency and Chronic Fatigue Syndrome [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Omnitrope's Efficacy in Managing IBD in American Male Children: A Review [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Omnitrope Enhances Reproductive Health in American Males with Growth Hormone Deficiency [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Omnitrope Therapy Enhances Musculoskeletal Health in American Male Children [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Omnitrope: Enhancing Endocrine Function in American Males with Growth Hormone Deficiency [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Omnitrope's Role in Treating GHD and Fibromyalgia in American Males [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Omnitrope Therapy Enhances Urological Health in Growth-Deficient American Male Children [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Omnitrope's Efficacy in Managing Allergic Rhinitis in American Children [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Omnitrope: A Dual Approach to GHD and Psoriasis in American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Omnitrope Enhances Skin Health in American Males with Growth Hormone Deficiency [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Omnitrope Therapy: Enhancing Growth and Nutrition in Children with Growth Disorders [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Omnitrope's Impact on Geriatric Health in American Males with GHD [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Omnitrope: Managing Growth Hormone Deficiency and Eczema in American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Omnitrope's Role in Treating Anorexia Nervosa in American Males: Benefits and Risks [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Omnitrope's Potential in Treating GHD in American Males with Alzheimer's: A Review [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Omnitrope's Role in Treating GHD in American Males with Schizophrenia: Benefits and Considerations [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Omnitrope's Impact on Psychiatric Health in American Men with Growth Hormone Deficiency [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Omnitrope Therapy Enhances Bone Health in Geriatric American Males: A Comprehensive Overview [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Omnitrope's Role in Managing Growth and Motor Issues in Cerebral Palsy Children [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Omnitrope: A Vital Treatment for GHD in American Males Post-TBI [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Omnitrope's Role in Managing Growth Hormone Deficiency in Parkinson's Disease [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Omnitrope Enhances Recovery in American Males Post-Pituitary Surgery for GHD [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Omnitrope Therapy Enhances Rehabilitation in American Male Pediatric Patients [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]