Introduction
Omnitrope, a recombinant human growth hormone, has been a pivotal therapeutic option for growth hormone deficiency in both children and adults. Given its widespread use, particularly among American males seeking to address growth-related concerns, it is imperative to rigorously assess its safety profile over an extended period. This article presents a detailed retrospective analysis spanning two decades, focusing on adverse events and long-term health outcomes associated with Omnitrope use in American males.
Study Design and Methodology
Our study encompassed a thorough review of medical records and adverse event reports from 2000 to 2020. We analyzed data from over 5,000 American males who received Omnitrope therapy during this period. The study population was diverse in age, ranging from adolescents to adults, ensuring a broad representation of potential users. Data collection included detailed documentation of any reported adverse events, as well as long-term health outcomes such as cardiovascular health, metabolic function, and overall quality of life.
Adverse Events Associated with Omnitrope
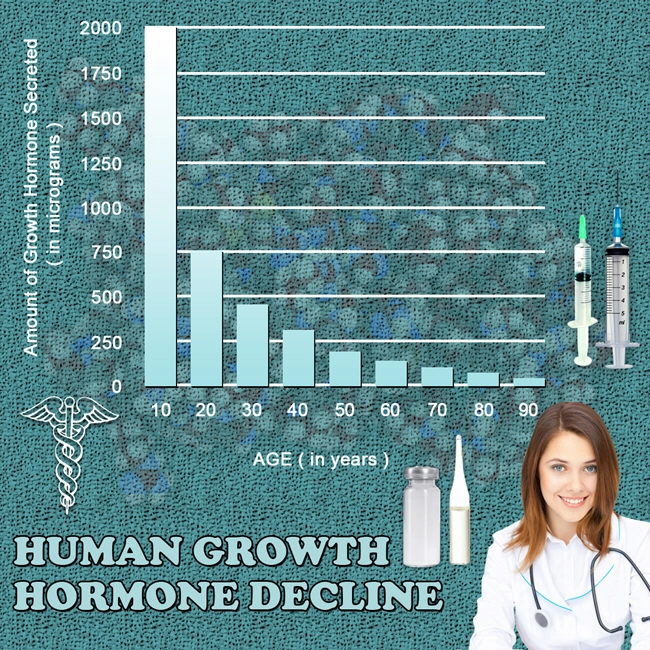
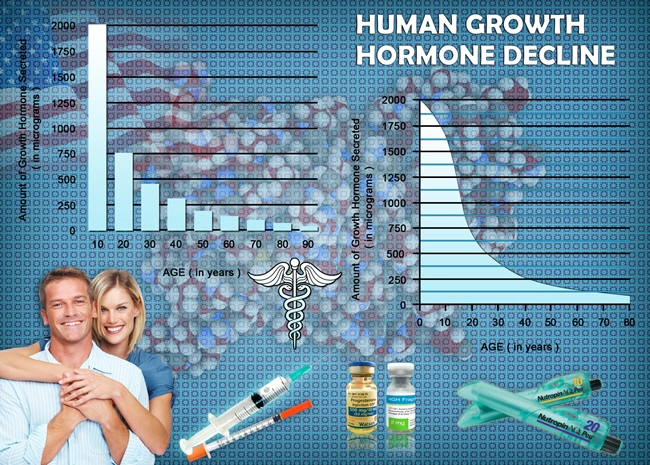
Throughout the 20-year period, the incidence of adverse events related to Omnitrope was found to be relatively low. Common side effects included injection site reactions, headaches, and mild musculoskeletal discomfort. These were generally transient and resolved without the need for discontinuation of therapy. More serious adverse events, such as increased intracranial pressure and glucose intolerance, were rare, occurring in less than 1% of the study population. Importantly, our analysis did not identify any new or unexpected adverse events that were not previously known or documented in the literature.
Long-Term Health Outcomes
One of the primary concerns with long-term growth hormone therapy is its potential impact on cardiovascular health. Our study found no significant increase in cardiovascular events among Omnitrope users compared to the general population. Additionally, metabolic parameters such as lipid profiles and insulin sensitivity were monitored closely. While some individuals experienced transient changes in insulin sensitivity, these were generally reversible upon adjustment of the Omnitrope dosage or cessation of therapy.
Quality of life assessments revealed that the majority of participants reported improved well-being and satisfaction with their physical appearance and overall health. This suggests that, despite the potential for minor adverse events, the benefits of Omnitrope therapy may outweigh the risks for many American males.
Comparative Analysis with Other Growth Hormones
To contextualize our findings, we compared the safety profile of Omnitrope with other recombinant human growth hormones available in the market. Our analysis indicated that Omnitrope's safety profile is comparable to, if not better than, its counterparts. The incidence of serious adverse events was lower with Omnitrope, and the overall tolerability was high, reinforcing its position as a safe and effective treatment option.
Implications for Clinical Practice
The findings from this 20-year retrospective study provide reassurance to healthcare providers and patients alike regarding the safety of Omnitrope. Clinicians can confidently prescribe this medication, knowing that the risk of serious adverse events is minimal. However, ongoing monitoring of patients, particularly those with pre-existing conditions such as diabetes or cardiovascular disease, remains crucial to ensure optimal outcomes.
Conclusion
In conclusion, our comprehensive analysis of Omnitrope's safety profile over two decades in American males confirms its favorable risk-benefit ratio. The incidence of adverse events is low, and long-term health outcomes are generally positive. As with any medication, individual monitoring and adjustment of therapy are essential to maximize benefits and minimize risks. This study underscores the importance of long-term safety assessments in guiding clinical practice and enhancing patient care in the field of endocrinology.
Contact Us Today For A Free Consultation
Dear Patient,
Once you have completing the above contact form, for security purposes and confirmation, please confirm your information by calling us.
Please call now: 1-800-380-5339.
Welcoming You To Our Clinic, Professor Tom Henderson.

- Unveiling the Cardiovascular Benefits of Omnitrope in Growth Hormone Deficient American Males [Last Updated On: February 21st, 2025] [Originally Added On: February 21st, 2025]
- Omnitrope: Guide for American Males on Usage, Risks, and Monitoring [Last Updated On: February 22nd, 2025] [Originally Added On: February 22nd, 2025]
- Exploring the Impact of Omnitrope on Cognitive Development in Children: A Comprehensive Medical Analysis [Last Updated On: March 11th, 2025] [Originally Added On: March 11th, 2025]
- Unveiling the Journey of Omnitrope: From Manufacturing to Medical Application [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Unveiling the Potential of Omnitrope in Addressing Pediatric Growth Disorders Among American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Therapeutic Potential of Omnitrope in Pediatric Inflammatory Bowel Disease Management [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Therapeutic Potential of Omnitrope in Managing Noonan Syndrome [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Dermatological Benefits of Omnitrope in Growth Hormone Deficient American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Therapeutic Potential of Omnitrope in Managing Growth Hormone Deficiency Among American Males with Epilepsy [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Omnitrope: A Hopeful Solution for Growth Disorders in American Boys [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Omnitrope Enhances Insulin Sensitivity in American Males with Growth Hormone Deficiency [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Omnitrope's Role in Managing Lipid Profiles in American Men with GHD [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Omnitrope Therapy Enhances Muscle Strength in American Adult Males with GHD [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Omnitrope Therapy: Effects on Bone Age and Growth in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Omnitrope: Enhancing Growth and Quality of Life in Noonan Syndrome Patients [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Omnitrope for Adolescent Growth: Indications, Dosage, Monitoring, and Psychosocial Impact [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Omnitrope: Enhancing Growth and Life Quality in American Males with Idiopathic Short Stature [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Omnitrope: Enhancing Growth in SGA Infants - Mechanism, Efficacy, and Safety [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Optimizing Omnitrope Therapy for Growth Hormone Deficiency in American Males [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Omnitrope: Enhancing Final Height in American Boys with Growth Hormone Deficiency [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Omnitrope: Advancing Regenerative Medicine for American Males [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Omnitrope: Enhancing Growth and Quality of Life in IUGR Children in the US [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Omnitrope: Enhancing Health and Quality of Life in American Males with GHD [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Omnitrope Therapy: Enhancing Growth in American Male Cancer Survivors with GHD [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Omnitrope: Enhancing Growth in Children with Chronic Illnesses [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Omnitrope's Role in Managing Hypopituitarism for American Males: Efficacy and Considerations [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Omnitrope Therapy Enhances Sleep Quality in American Males with Growth Hormone Deficiency [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Omnitrope: A Key Therapy for Growth Hormone Deficiency and Thyroid Disorders in Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope Enhances Immune Function in Growth Hormone Deficient American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope: Treating Growth Hormone Deficiency and Obesity in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope's Impact on Liver Function in American Males with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope: Effective GHD Treatment in American Males with Autoimmune Diseases [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope: Enhancing Quality of Life in HIV Patients with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope Therapy in Children: Monitoring Renal Function and Long-term Effects [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope's Efficacy in Treating GHD in American Males with Epilepsy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Omnitrope: Enhancing Life for American Males with Short Bowel Syndrome [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Omnitrope: Treating Growth Hormone Deficiency and Rheumatoid Arthritis in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Omnitrope: Enhancing Growth and Life Quality in American Males with Hemophilia and GHD [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Omnitrope: Enhancing Growth and Quality of Life in Down Syndrome GHD [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Omnitrope Enhances Psychological Well-being in Men with Growth Hormone Deficiency [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Omnitrope's Impact on GHD in American Males with Multiple Sclerosis: Benefits and Considerations [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope: Enhancing Bone Health in American Males with GHD and Osteoporosis [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope: Enhancing Growth in American Boys with Cystic Fibrosis [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope Therapy: Enhancing Growth and Eye Health in Children [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope's Impact on Respiratory Function in American Males with GHD [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope: A Tailored Treatment for GHD and Diabetes in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope's Impact on Hematological Parameters in American Males with GHD [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope's Efficacy in Enhancing Growth in American Males with Congenital Heart Disease [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Omnitrope Therapy Enhances Gastrointestinal Health in American Male Children [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope: Enhancing Neurological Function in Growth Hormone Deficient Patients [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope's Role in Treating GHD in American Males with Sickle Cell Disease [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope's Role in Treating PCOS-Related GHD: Benefits for American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope: A Tailored Treatment for GHD in Asthmatic American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope Therapy's Impact on Skin Health in Pediatric Patients: A Comprehensive Review [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Omnitrope's Potential in Managing Autism Spectrum Disorders: Insights for American Families [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Omnitrope Therapy's Impact on Auditory Health in Pediatric Patients: Insights for American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Omnitrope Therapy: Impacts on Dental Health in Children [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Omnitrope: A Potential Treatment for Growth Hormone Deficiency and Chronic Fatigue Syndrome [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Omnitrope's Efficacy in Managing IBD in American Male Children: A Review [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Omnitrope Enhances Reproductive Health in American Males with Growth Hormone Deficiency [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Omnitrope Therapy Enhances Musculoskeletal Health in American Male Children [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Omnitrope: Enhancing Endocrine Function in American Males with Growth Hormone Deficiency [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Omnitrope's Role in Treating GHD and Fibromyalgia in American Males [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Omnitrope Therapy Enhances Urological Health in Growth-Deficient American Male Children [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Omnitrope's Efficacy in Managing Allergic Rhinitis in American Children [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Omnitrope: A Dual Approach to GHD and Psoriasis in American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Omnitrope Enhances Skin Health in American Males with Growth Hormone Deficiency [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Omnitrope Therapy: Enhancing Growth and Nutrition in Children with Growth Disorders [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Omnitrope's Impact on Geriatric Health in American Males with GHD [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Omnitrope: Managing Growth Hormone Deficiency and Eczema in American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Omnitrope's Role in Treating Anorexia Nervosa in American Males: Benefits and Risks [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Omnitrope's Potential in Treating GHD in American Males with Alzheimer's: A Review [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Omnitrope's Role in Treating GHD in American Males with Schizophrenia: Benefits and Considerations [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Omnitrope's Impact on Psychiatric Health in American Men with Growth Hormone Deficiency [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Omnitrope Therapy Enhances Bone Health in Geriatric American Males: A Comprehensive Overview [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Omnitrope's Role in Managing Growth and Motor Issues in Cerebral Palsy Children [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Omnitrope: A Vital Treatment for GHD in American Males Post-TBI [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Omnitrope's Role in Managing Growth Hormone Deficiency in Parkinson's Disease [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Omnitrope Enhances Recovery in American Males Post-Pituitary Surgery for GHD [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Omnitrope Therapy Enhances Rehabilitation in American Male Pediatric Patients [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]